New Drug Pipeline Monitor – December 2013
ISSN 2292-3136
Catalogue number: H79-5/2014E-PDF
About the PMPRB
The Patented Medicine Prices Review Board (PMPRB) is an independent quasi-judicial body established by Parliament in 1987.
The PMPRB has a dual role: to ensure that prices at which patentees sell their patented medicines in Canada are not excessive; and to report on pharmaceutical trends of all medicines and R&D spending by patentees.
The PMPRB reports annually to Parliament, through the Minister of Health, on its activities, on pharmaceutical trends relating to all medicines, and on R&D spending by patentees.
The NPDUIS Initiative
The National Prescription Drug Utilization Information System (NPDUIS) provides critical analyses of drug price, utilization, and cost trends in Canada to support drug plan policy decision-making for participating federal, provincial, and territorial governments.
The NPDUIS initiative is a partnership between the PMPRB and the Canadian Institute for Health Information. It was established in 2001 by the federal/provincial/territorial Ministers of Health.
Acknowledgements
This report was prepared by the Patented Medicine Prices Review Board (PMPRB) under the provisions of the National Prescription Drug Utilization Information System (NPDUIS).
The PMPRB would like to acknowledge the following contributors:
- The members of the NPDUIS Steering Committee, for their expert oversight and guidance in the preparation of this report.
- Greg McComb, Senior Economist, NPDUIS, PMPRB, for his contribution to the analytical content of the report, as well as the PMPRB scientific and editing groups.
- Patricia Carruthers-Czyzewski, BScPhm, MSc, Sintera Inc., for providing pharmaceutical expertise in the development of the methodology and the scientific input of the report.
Disclaimer
NPDUIS is a research initiative that operates independently of the regulatory activities of the Board of the PMPRB. The statements and opinions expressed in this NPDUIS report do not represent the official position of the PMPRB.
1. Introduction
This is the fifth edition of the New Drug Pipeline Monitor (NDPM), a publication that provides information on drugs currently under development that may have an impact on federal, provincial and territorial (F/P/T) drug plan expenditure. Each report contains a list of pipeline drugs identified as part of a search of the BioPharm Insight® database1; a specialized database that provides information on over 21,000 drugs in clinical trials. The search is supported by a review of pharmacy literature, with a focus on Canadian studies.
Only drugs that meet a set of selection criteria are candidates for the NDPM. The selection criteria were prepared by Sintera Inc. for the PMPRB and were approved by the NPDUIS Steering Committee in 2006.2 This standardized approach is applied consistently to all editions of the NDPM. The criteria include: the phase of development, the indication, the mechanism of action and the impact on clinical practice. A decision-tree algorithm was developed so that criteria could be applied in a consistent, step-wise manner. Once a preliminary list is screened in, an effort is made to include pipeline drugs from a diverse set of therapeutic classes covered by public drug plans. In particular, consideration is given to high-cost drugs and classes where a new drug could have a financial impact, along with classes with a high utilization share of generic drugs.
As with previous reports, this edition of the NDPM updates the status of pipeline drugs identified in the previous editions. Some drugs were removed from the list either because they have entered the Canadian market or because the manufacturer is no longer conducting clinical trials. Similarly, drugs were retained if ongoing trials supported the initial assessment for inclusion.
This edition is organized into six sections. Following the Introduction, Section 2 provides an overview of the criteria used for drug selection, while Section 3 describes the algorithm used to apply the criteria. Section 4 discusses the BioPharm Insight database search and literature review, and provides a list of drugs identified for this report. Section 5 provides updates of the pipeline drugs identified in previous reports, while Section 6 lists the references cited in the report.
2. Criteria for Drug Selection
This section provides a brief description of the criteria used to select pipeline drugs.
2.1 Phase of Development
Only drugs in Phase III clinical trials are considered as potential candidates for the NDPM. Phase III clinical trials are the last step before a regulatory submission, and test the effectiveness and safety of a new drug with large, randomized patient groups. Drugs reaching this stage are more likely to proceed to market in the near future. Drugs in earlier phases of development may not necessarily progress beyond these stages.
2.2 Indication and Therapeutic Area
Drugs are considered to be potential candidates if they could be used to treat life-threatening conditions, conditions with unmet needs or rare diseases, or if they could potentially change clinical practice in a therapeutic area.
2.3 Drug Description
Drug description keywords that flag that a new drug could potentially change clinical practice include: first drug in a class, different mechanism of action, novel technology, add-on therapy, targeted niche, or an existing drug with a new indication.
2.4 Clinical and Other Impacts
Drugs must demonstrate the potential to have a significant clinical impact or a significant impact on other sectors of the health care system. Examples include: increased efficacy versus existing drugs; impacts on patient health, such as increased life expectancy or quality of life; new or redefined outcomes; or an improved safety profile.
3. Methodology for Applying Criteria
The main source of information for the NDPM is the BioPharm Insight database, which tracks drugs from pre-clinical discovery through clinical trials to market launch and subsequent sales.
The database is a comprehensive resource on investigational drugs and, at any one time, may contain more than 21,000 drugs. The database search allows drugs to be selected under various fields, including phase of development, therapeutic area, indication, drug mechanism, orphan drug, fast track and molecule type.
An algorithm developed for selecting drugs is illustrated in Figure 1. The algorithm combines the search capabilities of the BioPharm Insight database and the key criteria used to identify potentially high-impact drugs. Because the sources of information for this database are largely from the US, additional sources are used to determine whether the new drugs are in development in Canada.
Figure 1. Algorithm to select drugs for the New Drug Pipeline Monitor
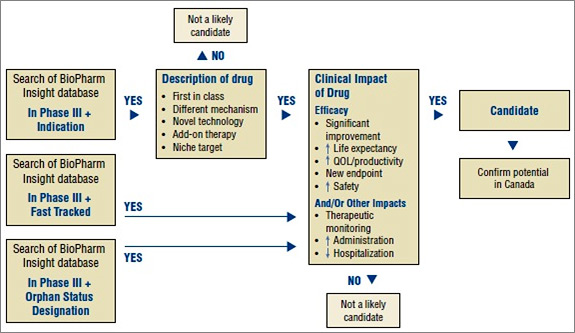
As the first step in identifying potential candidates for the NDPM, the BioPharm Insight database is searched for drugs currently in Phase III development. Phase III trials may have just been initiated for these drugs or some Phase III results may be available. Drugs in Phase III development are then screened by therapeutic area and indication. Drugs are considered as potential candidates for the NDPM if they have been identified by the US Food and Drug Administration (FDA) as orphan drugs, which treat rare diseases, or fast-track drugs. The FDA considers drugs for fast-track development if they are intended to treat serious or life-threatening conditions or if they demonstrate the potential to address unmet medical needs.
For drugs that are neither fast tracked nor designated as orphan drugs, the drug profiles are searched for keywords relating to specific drug descriptions, such as first-in-class, different mechanism, novel technology, add-on therapy, targeted niche or existing drugs with a new indication. If the drugs have these key descriptors, Phase III results are scanned to further validate the drug characteristics identified in the profile, such as a significantly increased efficacy or increased safety. At this point, drugs just entering Phase III trials are screened out, since there is insufficient information to make a scientific assessment.
Next, Canadian sources are consulted to determine whether there is information on any Canadian development. The main source of information is Pharmacy Practice, which publishes an annual list of promising new drugs in the later stages of development (Phase III or beyond) in Canada. This is followed by a scientific assessment of the identified drugs. If the preliminary Phase III results suggest an efficacy/safety impact, the drugs are then considered for inclusion in the NDPM. Finally, to confirm the selection of new drugs for the NDPM, consideration is given to the likelihood of coverage by F/P/T public drug plans, based on indication and form.
4. Identification of Candidates for the New Drug Pipeline Monitor
4.1. BioPharm Insight database search
The first step in identifying potential candidates was a search of the BioPharm Insight database. As in previous analyses, only drugs currently in Phase III development or under review by the FDA (i.e., New Drug Application (NDA) or Biological License Application (BLA) filed) were screened in. The search for drugs was conducted from September 3 to 13, 2013, in accordance with the algorithm described in the previous section.
The Biopharm Insight database is updated on an ongoing basis: some data is updated every few hours, while other sections are updated weekly as the information is refreshed by the source.1 Just before publication, the status of drugs in the NDPM was checked against the updated information in the Biopharm Insight database.
Table 1 summarizes the results of this search by therapeutic area. The database profile for each of these drugs was reviewed, with particular attention given to the drug description field. Specific keywords were sought, such as first-in-class or different mechanism. If these keywords were identified, the next step was to determine the results, if any, of Phase III clinical trials. Under the development history field of the drug profile, details of the Phase III results were scanned to further validate drug characteristics, such as increased efficacy or safety. If this scan revealed a lack of effect or a safety issue, the drug was screened out.
Table 1. BioPharm Insight search results by therapeutic class
| Therapeutic area |
Number of "hits" |
| In Phase III |
NDA/BLA filed |
| Cancer |
213 |
36 |
| Cardiovascular |
58 |
34 |
| Central nervous system |
64 |
62 |
| Dermatology |
36 |
9 |
| Eye and ear |
36 |
10 |
| Gastrointestinal |
47 |
33 |
| Genitourinary |
34 |
22 |
| Hematological |
46 |
26 |
| HIV infections |
9 |
8 |
| Hormonal system |
76 |
33 |
| Immune system |
77 |
33 |
| Infectious diseases |
105 |
69 |
| Musculoskeletal |
54 |
10 |
| Nephrology |
10 |
5 |
| Pain |
54 |
35 |
| Respiratory |
31 |
19 |
| Total* |
950 |
444 |
Table 2 is a working list of drugs that were screened in for this step. Biologics are identified separately, as they tend to be high-cost drugs with the potential to impact drug plans. Of the 199 drugs screened in, 51 were biologics. In most therapeutic areas, one or more biologics were identified, with the most 'hits' for cancer (24) and the immune system (5). Hormonal, infectious disease and musculoskeletal drugs each had 4 biologics in Phase III clinical trials and NDA/BLA filed.
Table 2. Specific drugs screened in by therapeutic area
| Therapeutic area |
Number of "hits" |
| In Phase III |
NDA/BLA filed |
| Chemical |
Biologic |
Chemical |
Biologic |
| Cancer |
41 |
23 |
16 |
1 |
| Cardiovascular |
5 |
1 |
5 |
0 |
| Central nervous system |
5 |
1 |
7 |
0 |
| Dermatology |
1 |
3 |
2 |
0 |
| Eye and ear |
0 |
0 |
1 |
1 |
| Gastrointestinal |
1 |
0 |
4 |
0 |
| Genitourinary |
2 |
0 |
1 |
0 |
| Hematological |
3 |
2 |
2 |
0 |
| HIV infections |
1 |
0 |
1 |
0 |
| Hormonal system |
4 |
3 |
8 |
1 |
| Immune system |
5 |
3 |
2 |
2 |
| Infectious diseases |
6 |
3 |
8 |
1 |
| Musculoskeletal |
5 |
4 |
3 |
0 |
| Nephrology |
3 |
1 |
0 |
0 |
| Pain |
0 |
0 |
2 |
0 |
| Respiratory |
0 |
1 |
4 |
0 |
| Total: in Phase III with NDA/BLA filed |
82 |
45 |
66 |
6 |
| Total |
199 |
To narrow the list further, all drugs screened in were checked against the most recent pipeline list in Pharmacy Practice3 to determine whether there was information on Canadian development. The next step was a scientific assessment of this preliminary list. For this assessment, details of the Phase III results from the BioPharm drug profiles were reviewed, specifically looking for significant improvements in efficacy and safety outcomes. In addition, the MEDLINE® database was searched to gain a sense of how the drug was viewed in the published literature.
As part of the final screening, an effort was made to include pipeline drugs from a diverse set of therapeutic classes covered by public drug plans. The potential financial impact on public drug plans was also taken into consideration. New drugs entering a class with high utilization (e.g., cardiovascular) or costly drugs (e.g., cancer) can be expected to increase the overall expenditure for a drug plan. The same logic can be applied to drugs entering therapeutic classes with a high utilization share of generic drugs. For example, numerous high-expenditure lipid-lowering medications (atorvastatin, simvastatin and pravastatin) have lost patent protection and have been replaced with less expensive generics. If a new drug with a significant therapeutic advancement enters this market, it has the potential to be an important cost driver.
4.2 Drugs Added to the New Drug Pipeline Monitor
Table 3 lists new candidates for the NDPM. Each drug met the selection criteria outlined in Section 3. The table lists the drug's trade name, company, therapeutic area and rationale for inclusion in the NDPM.
As in previous screening steps, biologics were identified; 2 of the 12 drugs added to this edition of the NDPM belong to this class. They are highlighted in the table for ease of identification.
Table 3. Drugs added to the New Drug Pipeline Monitor
| Drug (Trade name)* – Companies** |
Therapeutic area (ATC) – Indication |
Rationale for inclusion in the NDPM |
| Cancer |
|
Sotatercept
Acceleron Pharma; Celgene Corporation
|
Cancer (L01)
Anemia, Breast Cancer
|
- In Phase III trials
- Potential to stimulate bone formation: unmet medical need in treatment of bone loss
- Clinical studies ongoing to explore the potential to treat anemia and diseases of ineffective erythropoiesis are ongoing
- Administered as a subcutaneous injection
- An activin receptor ligand trap that reverses bone loss and reduces the degree of osteoporosis4
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
|
Tavocept
BioNumerik Pharmaceuticals;
Takeda Pharmaceutical Company Limited
|
Cancer (L01)
Add-on therapy to prevent cytotoxicity
|
- In Phase III trials
- FDA has granted Fast Track development designation
- Aimed at preventing common and serious side effects, particularly nerve and kidney damage, caused by treatment with taxanes and platinum drugs, which are widely used for treating cancer
- Add-on intravenous therapy: in combination with paclitaxel and cisplatin5
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
|
Vatalanib
Bayer AG; Novartis AG
|
Cancer (L01)
Colorectal cancer
|
- In Phase III trials
- Potentially first oral tyrosine kinase inhibitor to be used long-term in combination with standard chemotherapy for the treatment of patients with metastatic colorectal cancer.
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
| Cardiovascular |
|
LCZ696
Novartis AG
|
Cardiovascular (C09)
Hypertension, heart failure
|
- In Phase III trials for hypertension and Phase II for heart failure
- If Phase III trials show improved clinical outcomes, it could become the new standard of care; current standard of care in heart failure is ACE inhibitors (e.g., enalapril)
- Oral combination drug with a component that is first in class: angiotensin receptor neprilysin inhibitor (ARNI)
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
|
Mipomersen (Kynamro)
Genzyme Corporation, a Sanofi Company; Isis Pharmaceuticals, Inc.
|
Antilipidemic agent (C10)
Hypercholesterolemia
|
- Approved by the FDA in January 2013
- First-in-class: an oligonucleotide inhibitor of apolipoprotein B-100 synthesis
- Potentially significant population: “Although the use of mipomersen is limited to severe familial hypercholesterolemia as a replacement for LDL-apheresis, it is likely to be more widely prescribed in patients at high risk for cardiovascular disease, especially those who are resistant to or intolerant of high-intensity statin therapy"6
- “Significantly reduced LDL-C, apolipoprotein B, and Lp(a) in hypercholesterolemic patients with, or at risk for, CHD not controlled by existing therapies”7
- Weekly subcutaneous injections
- Indicated as an adjunct to lipid-lowering medications and diet for patients with homozygous familial hypercholesterolemia
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
| Central Nervous System |
|
Edivoxetine
Eli Lilly & Co.
|
Central Nervous System (N06)
Depression;
attention deficit hyperactivity disorder (ADHD)
|
- In Phase III trials
- An oral, highly selective norepinephrine transporter reuptake inhibitor
- Significant population: it is being studied as an add-on therapy for major depressive disorder and as a monotherapy for pediatric attention deficit hyperactivity disorder (ADHD)
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
| Eye/Ear |
|
Aflibercept (Eylea)
Bayer AG; Regeneron Pharmaceuticals, Inc.; Santen Pharmaceutical Co., Ltd.
|
Ophthalmology (S01)
Macular degeneration
Biologic
|
- Approved by the FDA
- Intravitreal injection
- Vascular endothelial growth factor (VEGF) inhibitor
- Comparable efficacy to ranibizumab (Lucentis) but is administered at 8-week intervals, which indicates the potential to reduce the risk of monthly intravitreal injections and the burden of monthly monitoring8
- Switching therapy to aflibercept is effective for patients with polypoidal choroidal vasculopathy who develop tachyphylaxis to ranibizumab9
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
| Gastrointestinal |
|
Lorcaserin hydrochloride (Belviq)
Arena Pharmaceuticals, Inc.; CY Biotech Company Ltd.; Eisai Co., Ltd.; Ildong Pharmaceutical Co., Ltd.
|
Gastrointestinal (A08)
Obesity
|
- Approved by the FDA in June 2012
- New therapeutic class
- Significant population; high demand
- An oral, serotonin (5-hydroxytryptamine, 5-HT) 5-HT2C receptor agonist that that regulates food intake
- “Cardiovascular outcome data will be invaluable in determining lorcaserin's eventual utilization and place in therapy”10
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
| Genitourinary |
|
NX-1207
Nymox Pharmaceutical Corporation; Recordati S.P.A.
|
Genitourinary (G04)
Benign prostatic hyperplasia
|
- In Phase III trials
- First-in-class for the treatment of benign prostatic hyperplasia (BPH) a disorder that causes difficulties with urination associated with aging
- Administered as an office-based procedure by transrectal intraprostatic injection under ultrasound guidance
- “In four US clinical trials to date, NX-1207 has shown evidence of symptomatic improvement substantially better than currently approved BPH medications with no significant safety issues”11
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
| Hematological / Infectious Disease |
|
Alisporivir
Debiopharm Group; Novartis AG
|
Anti-infective (J05)
AIDS; hepatitis C
|
- In Phase III trials for AIDS and Phase II for hepatitis C
- First-in-class host-targeting antiviral: cyclophilin inhibitor (CPIs)
- Injectable form
- Good candidate for interferon-sparing combinations12
- Investigational in Canada (listed in Pharmacy Practice 2013)
|
| Musculoskeletal |
|
Ataluren
Genzyme Corporation, a Sanofi Company; PTC Therapeutics, Inc.; Sanofi
|
Gene therapy
Cystic Fibrosis
|
- In Phase III trials
- FDA has granted Fast Track designation
- An oral drug that permits ribosomes to read through premature stop codons in mRNA to produce functional protein
- “Correction of the underlying gene effect a particularly exciting prospect as a new therapy for Cystic Fibrosis”13
|
| Respiratory |
|
Lebrikizumab
Chugai Pharmaceutical Co., Ltd; Genentech, Inc.; Roche
|
Respiratory (R07)
Asthma
Biologic
|
- In Phase III trials
- Injection
- First biological for the treatment of asthma
- An interleukin (IL)-13 inhibitor that demonstrates benefits in patients with poorly controlled asthma
- “IL-13 antagonists may fulfill an important unmet need in patients with poorly controlled asthma and biologic evidence of persistent IL-13 activity.”14
- A targeted therapy for patients with higher levels of serum periostin, which is involved in subepithelial fibrosis, raising the possibility of altering airway remodeling over the long term15
- Investigational status in Canada (listed in Pharmacy Practice 2013)
|
*If the drug and trade name are the same, only one entry is made.
** Companies 'working on a drug' as defined by the BioPharm Insight® database. More than one company may develop and market a drug, and their relationship may be defined by a licensing agreement.
Abbreviation: FDA, Food and Drug Administration.
5. Status Updates
5.1. Drugs Retained in the Pipeline List
Table 4 lists the drugs from previous editions of the NDPM that remain as pipeline candidates. A status update based on recent scientific literature is provided, along with a rationale for retaining the drugs on the pipeline list.
Table 4. Drugs retained in pipeline list
| Drug (Trade name) – Company |
Therapeutic area (ATC) – Indication |
Status update and rationale for
retaining in the pipeline list |
|
Agomelatine (Valdoxan)
Les Laboratoires Servier;
Novartis AG
|
Antidepressants (N06A)
Major depression
|
Previous description:
- Approved by the FDA and EMA (BioPharm Insight); not marketed in the US16
- First in a new class of antidepressants with a unique mechanism of action: melatonergic antidepressant
Update:
- “As agomelatine has a mechanism of action that differs from other agents, it may represent a valuable additional treatment option in those patients who do not respond fully or who do not tolerate the side effects of other antidepressants.”17
Rationale: Current literature continues to suggest that agomelatine is an important new approach to depression with at least comparable, and maybe superior, efficacy and fewer side effects.
|
|
Albiglutide (Syncria)
GlaxoSmithKline plc;
Human Genome Sciences, Inc.
|
Diabetes (A10) and Cardiac Therapy (C01)
Type 2 diabetes and heart failure
Biologic
|
Previous description:
- Regulatory filing for FDA approval for type 2 diabetes; not marketed in the US16
- Phase II trials ongoing for heart failure (BioPharm Insight database)
- Subcutaneous injection; a long-lasting (once weekly) glucagon-like peptide-1 agonist
- Overall higher treatment satisfaction for patients because of ease of use and need for less frequent dosing18
Update:
- Still listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that albiglutide is effective and may improve compliance through ease of use.
|
|
Cetilistat (Cametor)
Norgine BV;
Takeda Pharmaceutical Company Limited
|
Antiobesity (A08)
Obesity
|
Previous description:
- Phase III trials complete in US for obesity; New Drug Application (NDA) submitted in Japan
- Showed similar weight loss to that seen with orlistat (Xenical) but with up to 90% fewer severe GI side effects;19 could provide a better alternative in a significant population
Update:
- Approved by the FDA (BioPharm Insight)
- Described as “an emerging therapy for obesity”20
- No longer listed as Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Although there is limited new published literature, it has the potential to be a better tolerated alternative for obesity treatment.
|
|
Darapladib
DiaDexus LLC;
GlaxoSmithKline plc;
Human Genome Sciences, Inc.
|
Cardiac Therapy (C01)
Atherosclerosis
|
Previous description:
- New class: inhibitor of lipoprotein associated phospholipase A2 (Lp-PLA2)
- Different mechanism of action for treatment of atherosclerosis compared to statins
- Decreases cardiovascular risk; inhibits formation of atherosclerotic plaques; has the potential to affect patient outcomes (myocardial infarction, stroke, cardiovascular death)21
Update:
- Still listed as an Investigational drug in Canada (Pharmacy Practice 2013)
- Phase III trials are expected to be completed in November 2014.22
Rationale: Current literature continues to suggest that darapladib could be an important pipeline drug.
|
|
Ecallantide (Kalbitor)
Dyax Corporation;
Sigma-Tau Pharmaceuticals
|
Antihemorrhagics (B02)
Hereditary angioedema (HAE)
Biologic
|
Previous description:
- Unmet medical need: first specific therapy for acute attacks – HAE is a debilitating, potentially fatal disease
- Injectable (IV and SC; intravitreal)
Update:
- Combined data from four clinical studies (EDEMA2, EDEMA3, EDEMA4 and DX-88/19) demonstrated that ecallantide 30 mg administered SC was effective for treatment of laryngeal HAE attacks;23 also shown to be effective for HAE attacks in adolescents.24
- Not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that ecallantide fills a medical need for treatment of acute HAE.
|
|
Ispronicline
AstraZeneca PLC;
Targacept, Inc.
|
Psycholeptics (N05) and Psychoanaleptics (N06)
Alzheimer's disease; attention deficit hyperactivity disorder; depression/stress and anxiety
|
Previous description:
- Phase II trials for Alzheimer's disease and attention deficit hyperactivity disorder; Phase III trials for depression/stress and anxiety
Achieved statistically significant results on all of the primary endpoints, reflecting improved cognitive performance by memory-impaired older adults
Update:
- Not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that ispronicline could be an effective treatment for Alzheimer's disease.
|
|
Istradefylline
Kyowa Hakko Kirin Pharma, Inc;
Valeant Pharmaceuticals International, Inc.
|
Anti-Parkinson Drugs (N04)
Parkinson' disease
|
Previous description:
- First in a new class: selective adenosine A2A receptor antagonist
- Impacts on disease progression rather than treating symptoms
Update:
- Approved as adjunctive therapy in Japan (2013)
- Filed for approval is US (BioPharm Insight database)
- Not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that istradefylline may be a promising non-dopaminergic therapy for the treatment of Parkinson' disease.
|
|
Laquinimod
Active Biotech AB;
Teva Pharmaceutical Industries Ltd.
|
Immunostimulants (L03) and Immunosuppressants (L04)
Multiple sclerosis, Crohn's disease and lupus
Biologic
|
Previous description:
- Regulatory filing for FDA approval for multiple sclerosis; Phase II trials ongoing for Crohn's disease and lupus (BioPharm Insight database)
- Novel once-daily, orally administered immunomodulatory compound
Update:
- Published studies demonstrate beneficial effects of laquinimod (0.6 mg/day) on clinical and neuroimaging surrogate markers in adult patients with relapsing–remitting multiple sclerosis (RRMS) with a favourable risk–benefit profile; in particular, there is no evidence for an increased risk of cardiac adverse events.25
- Still listed as Investigational drug status in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that laquinimod may be a promising therapy for the treatment of RRMS.
|
|
Macitentan (Opsumit)
Actelion Pharmaceuticals Ltd.
|
Cardiac Therapy
(C01)
Pulmonary arterial and digital ulcers
|
Previous description:
- Phase III trials ongoing for digital ulcers; approved by FDA approval for pulmonary arterial hypertension (PAH)
- An orally active dual endothelin receptor antagonist that improves long-term outcome in cardiovascular conditions
Update:
- In a study of 250 patients with symptomatic PAH, macitentan significantly reduced morbidity and mortality among patients with PAH vs. placebo26
- Still listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that macitentan may an effective treatment for pulmonary arterial hypertension.
|
|
Nintedanib
Boehringer Ingelheim GmbH
|
Cancer (L01)
Non-small cell and ovarian cancer
Biologic
|
Previous description:
- Phase III trials ongoing for ovarian and non-small cell cancer (BioPharm Insight database)
- A triple angiokinase inhibitor that targets three growth factor receptors: vascular endothelial, platelet-derived and fibroblast
Update:
- Filing for FDA approval for non-small cell cancer (BioPharm Insight database)
- May have a role in idiopathic pulmonary fibrosis (IPF), a rare, life-threatening disease27
- Still listed as an Investigational drug in Canada (Pharmacy Practice Dec 2013)
Rationale: Current literature continues to suggest that nintedanib may have a role to play in lung cancer. It may also be useful in idiopathic pulmonary fibrosis.
|
|
Phenoxodiol
Marshall Edwards, Inc.;
Novogen Limited
|
Cancer (L01)
Ovarian, cervical, head and neck, kidney, leukemia, prostate cancer
|
Previous description:
- FDA has granted Fast Track status to its development phenoxodiol as a chemo-sensitizer for platinum and taxane drugs
- Has demonstrated improved survival in difficult to treat cancers
Update:
- Not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
- Still listed in Phase III trial (BioPharm Insight database)
Rationale: Although current literature is limited, it has the potential to improve survival.
|
|
Ponesimod
Actelion Pharmaceuticals Ltd; Roche
|
Immunostimulants (L03) and Immunosuppressants (L04)
Multiple sclerosis and psoriasis
Biologic
|
Previous description:
- Phase III trials ongoing for psoriasis; Phase II trials for multiple sclerosis (BioPharm Insight database)
- Potential for once-a-day oral dosing, for multiple autoimmune disorders28
Update:
- Still listed as Investigational drug status in Canada (Pharmacy Practice 2013)
Rationale: Although current literature is limited, ponesimod is likely to be an important therapy for multiple sclerosis.
|
|
Rebamipide (Mucosta)
Acucela, Inc.;
Novartis AG;
Otsuka Pharmaceutical Co., Ltd.
|
Ophthalmological Preparations (S03)
Dry eye syndrome
|
Previous description:
- Phase III clinical trials in US (BioPharm Insight database); approved for dry eye syndrome in Japan
- There is a need for effective therapy for dry eye that treats the underlying cause of the syndrome
- Large population: 22 million patients visit an ophthalmologist worldwide for dry eye symptoms
Update:
- “Rebamipide administered to treat the short break-up time type of dry eye significantly improved optical quality because of the improvement in tear stability.”29
- Not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that repamipide is effective in the treatment of dry eye.
|
|
Safinamide
Meiji Seika Pharma Co., Ltd.;
Merck Serono SA;
Newron Pharmaceuticals;
Zambon Group)
|
Anti-Parkinson Drugs (N04) and Antiepileptics (N03)
Parkinson's disease, epilepsy and restless leg syndrome
|
Previous description:
- Multiple mechanisms of action and multiple indications
- Significant population
- If efficacy is improved over existing agents, could replace inexpensive alternatives, such as quinine in restless leg syndrome
Update:
- Regulatory filing for Parkinson's Disease expected in the fourth quarter, 2013 (Biopharm Insight Database)
- Studies in Parkinson's disease (PD) are promising: “Safinamide has the potential to become an important compound for the therapy of PD, since its symptomatic efficacy appears to be superior to available monoamine oxidase-B inhibitors or N-methyl-d-aspartate receptor antagonists like amantadine, according to available trial outcomes”30
- No longer listed as an Investigational drug in Canada (Pharmacy Practice 2103)
Rationale: Current literature continues to suggest that safinamide may an effective treatment for Parkinson's disease.
|
|
Satraplatin
Agennix AG;
Celgene Corporation;
GPC Biotech AG;
Spectrum Pharmaceuticals, Inc.
|
Cancer (L01)
Prostate cancer and small-cell lung cancer
|
Previous description:
- First oral platinum compound
- Fast Track designation in US as a second-line chemotherapy treatment for prostate cancer; Filing for FDA approval for prostate cancer with Phase III trials for general cancer and Phase II trials for small-cell lung cancer
Update:
- “Given the favorable toxicity profile and convenient oral administration, satraplatin may warrant development in settings that preclude cisplatin, for example, underlying renal dysfunction, elderly age and poor performance status.”31
- Phase II studies have been published32, 33
- Not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Although current literature suggests that satraplatin may not increase survival, if it is the first oral platinum compound approved, it will have an impact on clinical practice.
|
|
Selexipag
Actelion Pharmaceuticals Ltd;
Nippon Shinyaku Co., Ltd
|
Antihypertensives (C02)
Pulmonary arterial hypertension
|
Previous description:
- Oral, long-acting, selective prostacyclin receptor agonist for pulmonary arterial hypertension (PAH)
- In Phase III trials
Update:
- “The phase III trial – GRIPHON – has a clinically relevant and highly robust primary end-point of time to first morbidity/mortality event and will provide vital information on the long-term effects of selexipag in patients with PAH”34
- Still listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that selexipag may an effective treatment for pulmonary arterial hypertension.
|
|
Serelaxin (Relaxin)
Corthera Inc.;
Novartis AG;
Paladin Labs, Inc.
|
Cardiac Therapy (C01)
Heart failure
Biologic
|
Previous description:
- In Phase III trials
- Hormone-based injectable drug
- Constitutes a major advance in acute heart failure (AHF) treatment since it addresses two of the most important aspects of treating AHF: hemodynamic improvement and renal protection35
Update:
- Some results have been published: “Treatment of acute heart failure with serelaxin was associated with dyspnea relief and improvement in other clinical outcomes, but had no effect on readmission to hospital. Serelaxin treatment was well tolerated and safe, supported by the reduced 180-day mortality”36
- Not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to suggest that serelaxin may an effective treatment for heart failure.
|
|
Tofacitinib (Xeljanz)
Pfizer Inc.;
Takeda Pharmaceutical)
|
Immunosuppres-sants (L04)
Numerous indications: ankylosing spondylitis, rheumatoid arthritis, Crohn's disease, psoriasis, transplants, others
|
Previous description:
- In Phase III trials
- Significant population: rheumatoid arthritis
- Oral dosage form in an area where injectable products are currently the standard of care
Update:
- Still listed as an Investigational drug in Canada (Pharmacy Practice 2013)
- “In patients with rheumatoid arthritis receiving methotrexate, tofacitinib was significantly superior to placebo and was similar to adalimumab (Humira) in efficacy.”37
Rationale: Current literature continues to supports that tofacitinib is an effective oral treatment for chronic inflammatory conditions.
|
|
Voclosporin (Luveniq)
SBio Inc.;
Iljin Life Science, Co., Ltd.;
Isotechnika, Inc.;
Lux Biosciences;
Paladin Labs, Inc.;
Roche
|
Ophthalmological Preparations (S03) and Immuno-suppressants (L04)
Uveitis, kidney and other transplantations; psoriasis
|
Previous description:
- Phase III trials for uveitis; filing for FDA approval for psoriasis; Phase III for kidney transplantation (BioPharm Insight database)
- Phase III Canadian study showed significantly improved quality of life for patients with psoriasis38
Update:
- “The limited data available indicate at least comparable results relative to current therapy with a better safety profile [in uveitis].”39
- No longer listed as an Investigational drug in Canada (Pharmacy Practice 2013)
Rationale: Current literature continues to supports that voclosporin is an effective oral treatment for uveitis.
|
Abbreviations: EMA: European Medicines Agency; FDA, Food and Drug Administration
5.2 Drugs Removed from Pipeline List
Tables 5 and 6 provide a list of drugs from previous editions of the NDPM that have been removed from the pipeline list. The drug in Table 5 was removed because the manufacturer was granted authority to market the drug in Canada. Health Canada grants that authority in a Notice of Compliance (NOC). Table 5 also provides information on recommendations made by the Canadian Drug Expert Committee (CDEC), which is an advisory body to the Canadian Agency for Drugs and Technologies in Health (CADTH).40 The CDEC makes recommendations to each of the participating federal, provincial, and territorial publicly funded drug plans regarding the listings on their formularies.
The Patented Medicine Prices Review Board (PMPRB) reviews prices of patented medicines to ensure they are not excessive. The drug in Table 5 is priced within the guidelines.
Table 6 lists drugs that have been removed because a scientific assessment no longer supports retention on the pipeline list. Reasons for removal include lack of information or subsequent clinical trials that cite insufficient efficacy or safety.
Table 5. Drugs removed from pipeline list: market authority granted by Health Canada
| Drug (Trade name) – Company |
Therapeutic area (ATC) – Indication |
NOC* date /Date of first sale** |
CADTH recommendation† |
PMPRB review |
|
Mirabegron (Myrbetriq)
Astellas Pharma Canada Inc.
|
Urologicals (G04)
Incontinence, overactive bladder
|
NOC granted 2013-03-06
Marketed in Canada as of 2013-03-2841
|
An embargo period for a final CADTH recommendation was extended to November, 2013. The recommendation was sent to drug plans and the manufacturer. |
Price within guidelines |
*A Notice of Compliance is issued by Health Canada and indicates that the drug product meets the regulatory requirements for use in humans or animals and that the product is approved for sale in Canada.
**The Date of first sale is as reported to the PMPRB. This date may precede the Health Canada NOC date, as a product may be sold under the Special Access Programme, the Clinical Trial Application or it is an Investigational New Drug.
†CADTH recommendations are made by the Canadian Drug Expert Committee (CDEC), an independent advisory body composed of individuals with expertise in drug therapy and drug evaluation. Submissions by manufacturers are voluntary.
Table 6. Drugs removed from pipeline list: scientific assessment
| Drug (Trade name) – Company |
Rationale for removal |
|
Sipuleucel-T (Provenge)
Dendreon Corporation
Biologic
|
- Although marketed in US, “due to its cost, complicated treatment regimen, and the large number of newly approved competing therapeutics to treat castrate-resistant prostate cancer (CRPC), the utilization of sipuleucel-T has been lower than forecasted. Furthermore, the observed increase in overall survival in the absence of a change in progression-free survival or PSA response presents a challenge for clinicians. Using traditional markers of response to treatment in CRPC, there is no evidence that sipuleucel-T exerts a measurable antitumor activity. Sipuleucel-T's impact on the natural history of the disease is somewhat perplexing as there is no evidence of tumor burden reduction after treatment with this immune compound. Without these efficacy biomarkers, it will be challenging to incorporate sipuleucel-T in the treatment protocols.”42
- Is not listed as an Investigational drug in Canada (Pharmacy Practice 2013)
|
6. References
1 BioPharm Insight® database. Available at: http://www.infinata5.com/BioPharm/AccessPoint.aspx?action=Login.ShowLogin&datakey=BioPharm (Accessed September 3–13, 2013).
2 While developing the NDPM methodology in 2006, the PMPRB consulted with the Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH is currently doing complementary horizon scanning work with the Canadian Network for Environmental Scanning in Health (CNESH). CADTH's work has a broader focus, and includes health care technologies, medical procedures as well as drugs.
3 Murdoch LA. Under investigation: A summary of drugs in late-stage development in Canada. March 4, 2013 for Pharmacy Practice. Available at: www.canadianhealthcarenetwork.ca/pharmacists/news/drug-news/under-investigation-19625 (Accessed September 13, 2013)
4 Iancu-Rubin C, Mosoyan G, Wang J, et al.Stromal cell-mediated inhibition of erythropoiesis can be attenuated by Sotatercept (ACE-011), an activin receptor type II ligand trap. Exp Hematol. 2013;41(2):155-166.e17.
5 Masuda N, Negoro S, Hausheer F, et al. Phase I and pharmacologic study of BNP7787, a novel chemoprotector in patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2011 Mar; 67(3):533-42.
6 Sahebkar A, Watts GF. New LDL-Cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin Ther. 2013;35(8):1082-98.
7 Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M. Mipomersen, An apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2013: S0735-1097(13)04044-8.
8 Semeraro F, Morescalchi F, Duse S, et al. Aflibercept in wet AMD: specific role and optimal use. Drug Des Devel Ther. 2013;7:711-22.
9 Miura M, Iwasaki T, Goto H. Intravitreal aflibercept for polypoidal choroidal vasculopathy after developing ranibizumab tachyphylaxis. Clin Ophthalmol. 2013;7:1591-5.
10 Berlie HD, Hurren KM. Evaluation of lorcaserin for the treatment of obesity. Expert Opin Drug Metab Toxicol. 2013;9(8):1053-9.
11 Shore N, Cowan B. The potential for NX-1207 in benign prostatic hyperplasia: an update for clinicians. Ther Adv Chronic Dis. 2011;2(6):377-83.
12 Flisiak R, Jaroszewicz J, Flisiak I, £apiñski T. Update on alisporivir in treatment of viral hepatitis C. Expert Opin Investig Drugs. 2012;21(3):375-82.
13 Jones AM, Helm JM. Emerging treatments in cystic fibrosis. Drugs. 2009 1;69(14):1903-10.
14 Corren J. Role of Interleukin-13 in asthma. Curr Allergy Asthma Rep. 2013 Sep 12.
15 Thomson NC, Patel M, Smith AD. Lebrikizumab in the personalized management of asthma. Biologics. 2012;6:329-35.
16 US Food and Drug Administration (FDA) approved drug products website. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Search_Drug_Name (Accessed September 13, 2013)
17 Smeraldi E, Delmonte D. Agomelatine in depression. Expert Opin Drug Saf. 2013 Sep 16.
18 Tzefos M, Harris K, Brackett A. Clinical efficacy and safety of once-weekly glucagon-like peptide-1 agonists in development for treatment of type 2 diabetes mellitus in adults. Ann Pharmacother. 2012;46(1):68-78.
19 Kopelman P, Groot Gde H, Rissanen A, et al. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity (Silver Spring). 2010;18(1):108-15.
20 George M, Rajaram M, Shanmugam E. New and emerging drug molecules against obesity. J Cardiovasc Pharmacol Ther. 2013 Sep 24.
21 White H. Editorial: why inhibition of lipoprotein-associated phospholipase A2 has the potential to improve patient outcomes. Curr Opin Cardiol. 2010; 25(4):299-301.
22 National Institute for Health Research, NIHR Horizon Scanning Centre. Darapladib for cardiovascular risk reduction in patients with coronary heart disease – add on therapy (NIHR HSC ID: 2237). May 2013.
23 Sheffer AL, MacGinnitie AJ, Campion M, et al. Outcomes after ecallantide treatment of laryngeal hereditary angioedema attacks. Ann Allergy Asthma Immunol. 2013;110(3):184-188.e2.
24 MacGinnitie AJ, Davis-Lorton M, Stolz LE, Tachdjian R. Use of ecallantide in pediatric hereditary angioedema. Pediatrics. 2013;132(2):e490-7.
25 Thöne J, Ellrichmann G. Oral available agents in the treatment of relapsing remitting multiple sclerosis: an overview of merits and culprits. Drug Healthc Patient Saf. 2013;5:37-47.
26 Pulido T, Adzerikho I, Channick R, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809-18.
27 Woodcock HV, Molyneaux PL, Maher TM. Reducing lung function decline in patients with idiopathic pulmonary fibrosis:potential of nintedanib. Drug Des Devel Ther. 2013;7:503-10.
28 Brossard P, Derendorf H, Xu J, et al. Pharmacokinetics and Pharmacodynamics of Ponesimod, a Selective S1P1 Receptor Modulator, in the First-in-Human Study. Br J Clin Pharmacol. 2013 Apr 18. doi: 10.1111/bcp.12129.
29 Koh S, Inoue Y, Sugmimoto T, et al. Effect of rebamipide ophthalmic suspension on optical quality in the short break-up time type of dry eye. Cornea. 2013;32(9):1219-23.
30 Müller T. Current status of safinamide for the drug portfolio of Parkinson's disease therapy. Expert Rev Neurother. 2013;13(9):969-77.
31 Doshi G, Sonpavde G, Sternberg CN. Clinical and pharmacokinetic evaluation of satraplatin. Expert Opinion on Drug Metabolism and Toxicology. 2012;8(1):103-11.
32 Figg WD, Chau CH, Madan RA, et al. Phase II study of satraplatin and prednisone in patients with metastatic castration-resistant prostate cancer: a pharmacogenetic assessment of outcome and toxicity. Clin Genitourin Cancer. 2013;11(3):229-37.
33 Vaishampayan UN, Fontana J, Heilbrun LK, et al. Phase II trial of bevacizumab and satraplatin in docetaxel-pretreated metastatic castrate-resistant prostate cancer. Urol Oncol. 2013 Feb 20. pii: S1078-1439(12)00423-1.
34 Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: the future is here. Eur Respir Rev. 2012 Dec 1;21(126):321-7.
35 Bani D, Bigazzi M. Relaxin as a cardiovascular drug: a promise kept. Curr Drug Saf. 2011 Nov 1;6(5):324-8.
36 Teerlink JR, Cotter G, Davison BA, et al; Relaxin in acute heart failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29-39.
37 van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508-19.
38 Kunynetz R, Carey W, Thomas R, et al. Quality of life in plaque psoriasis patients treated with voclosporin: a Canadian phase III, randomized, multicenter, double-blind, placebo-controlled study. Eur J Dermatol. 2011;21(1):89-94.
39 Schultz C. Voclosporin as a treatment for noninfectious uveitis. Ophthalmol Eye Dis. 2013;5:5-10.
40 The Common Drug Review database Available at: http://www.cadth.ca/en/products/cdr/search (Accessed November 11, 2013). [Only publicly available information is cited]
41 Health Canada Drug Product Database. Available at: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-eng.php. (Accessed September 13, 2013)
42 Acar O, Esen T, Lack N. New therapeutics to treat castrate-resistant prostate cancer. Scientific World Journal. 2013;2013:379641.