The PMPRB is responsible for reporting on trends in pharmaceutical sales and pricing for all medicines and for reporting research and development spending by patentees. In addition, the PMPRB undertakes studies and conducts analysis on a variety of topics related to pharmaceutical pricing and costs.
Trends in Sales of Patented Drug Products
Patentees are required under the Patented Medicines Regulations (Regulations) to submit detailed information on their sales of patented drug products, including quantities sold and net revenues received for each product by class of customer in each province and territory. The PMPRB uses this information to analyze trends in sales, prices and utilization of patented drug products.1 This section provides key statistical results from this analysis.
Sales and Prices
Canadians spend much more today on patented drug products than they did a decade ago, but it is important to understand that an increase in drug spending does not in itself imply rising drug prices. The PMPRB's Annual Reports from 1995 through 2003 noted that sales of patented drug products grew at annual rates consistently exceeding 10%, while average annual rates of change for prices were less than 1%. In these instances, sales growth was driven by changes in the volume and composition of drug utilization.
A variety of factors can produce such changes. These include:
- increases in total population
- changes in the demographic composition of the population (for example, shifts in the age distribution toward older persons with more health problems)
- increases in the incidence of health problems requiring drug therapy
- changes in the prescribing practices of physicians (for example, shifts away from older, less expensive drug products to newer, more expensive medications, or a shift toward higher or more frequent dosages)
- increases in the use of drug therapy instead of other forms of treatment
- the use of new drug products to treat conditions for which no effective treatment existed previously
Sales Trends
Table 7 reports patentees' total sales of patented drug products in Canada for 1990 through 2012. In 2012, sales of patented drug products declined to $12.8 billion from $12.9 billion in 2011, a decrease of 0.3%. By comparison, the annual growth in sales was 27.0% in 1999 and remained in double-digits until 2003.
The last column of Table 7 gives sales of patented drug products as a share of overall drug sales. This share rose from 43.2% in 1990 to a peak of 72.7% in 2003. It has generally declined since 2003, implying that sales of non-patented brand and generic drug products have grown faster than sales of patented drug products in recent years.
Table 7 Sales of Patented Drug Products, 1990–2012
| Year |
Patented drug products |
Sales of patented drug product share of all drug sales (%)* |
| Sales ($billions) |
Change (%) |
| 2012 |
12.8 |
-0.3 |
59.3 |
| 2011 |
12.9 |
4.0 |
58.6 |
| 2010 |
12.4 |
-3.8 |
56.0 |
| 2009 |
12.9 |
2.4 |
59.2 |
| 2008 |
12.6 |
2.4 |
61.7 |
| 2007 |
12.3 |
3.4 |
63.2 |
| 2006 |
11.9 |
3.5 |
67.8 |
| 2005 |
11.5 |
4.5 |
70.6 |
| 2004 |
11.0 |
7.8 |
72.2 |
| 2003 |
10.2 |
14.3 |
72.7 |
| 2002 |
8.9 |
17.5 |
67.4 |
| 2001 |
7.6 |
18.9 |
65.0 |
| 2000 |
6.3 |
16.7 |
63.0 |
| 1999 |
5.4 |
27.0 |
61.0 |
| 1998 |
4.3 |
18.9 |
55.1 |
| 1997 |
3.7 |
22.6 |
52.3 |
| 1996 |
3.0 |
12.8 |
45.0 |
| 1995 |
2.6 |
10.8 |
43.9 |
| 1994 |
2.4 |
-2.1 |
40.7 |
| 1993 |
2.4 |
9.4 |
44.4 |
| 1992 |
2.2 |
14.0 |
43.8 |
| 1991 |
2.0 |
13.1 |
43.2 |
| 1990 |
1.7 |
— |
43.2 |
* The denominator in this ratio comprises sales of patented, non-patented brand and generic drug products. Starting with the estimate for 2005, this value is derived from data contained in IMS Health's MIDAS database. In previous years, IMS data were used to calculate sales of generic drug products only, while sales of non-patented brand products were estimated from data submitted by patentees. This approach was abandoned because of anomalies related to year-to-year changes in the set of companies reporting to the PMPRB. Ratios reported for years before 2005 likely overstate the patented share, but by only a small amount. This small bias in no way invalidates the strong upward trend evinced by the results for the years 1990 through 2003.
Sources: PMPRB and MIDAS©, 2005-2012, IMS Health Incorporated or its affiliates. All rights reserved.2
Drivers of Sales Growth
Table 8 decomposes the sales growth that occurred between 2011 and 2012 into distinct elements reflecting the impacts of:
- previously patented drug products that have gone off-patent or left the Canadian market (“exiting drug effect”)
- patented drug products introduced to the Canadian market in 2012 (“new drug effect”)
- changes in prices among patented drug products with sales in Canada in both 2011 and 2012 (“price effect”)
- differences in the quantities of such drug products sold in the two years (“volume effect”)
- interactions of price and quantity changes (“cross effect”)
The first row of Table 8 gives these impacts as dollar amounts. The second row expresses the impacts as proportions of the overall change in sales between 2011 and 2012. For the sake of comparison, the third row provides average year-over-year proportionate impacts for 2007 through 2011.3
The results in this table show that the decline in sales that occurred between 2011 and 2012 was the result of drug products going off-patent; all other components contributed negatively toward the overall decrease in sales. The growth in new drugs, along with the price and volume effect, was not large enough to offset the negative effect of the exiting drugs on the overall sales.
Table 8 Decomposition of Changes in Sales of Patented Drug Products
|
Total change |
Exiting drug effect |
New drug effect |
Price effect |
Volume effect |
Cross effect |
| Sales impact, 2012/2011 ($millions) |
-27.25 |
-317.82 |
282.07 |
43.22 |
37.68 |
-72.40 |
| Proportion of total change, 2012/2011 (%) |
100.00 |
1,166.33 |
-1,035.13 |
-158.60 |
-138.29 |
265.69 |
| Average proportion of total change, 2007–2011 (%) |
100.00 |
168.02 |
-122.76 |
-21.94 |
38.27 |
38.40 |
Source: PMPRB
The pronounced decline in rates of sales growth over the last few years is a striking development. Figure 2 breaks down 2012 sales of patented drug products according to the year in which the product was first sold in Canada. Throughout the latter part of the 1990s and early 2000s, sales growth was largely driven by a succession of new “blockbuster” products that ultimately achieved very high sales volumes. Despite the recent patent expiries (patent cliff), these products still accounted for a substantial share of sales in 2012. Since the beginning of the 2000s, changes in the Canadian pharmaceutical environment, along with a reduction in the rate of introduction of new high-volume products, has resulted in decreased growth.
Figure 2
Share of 2012 Sales of Patented Drug Products by Year of Introduction
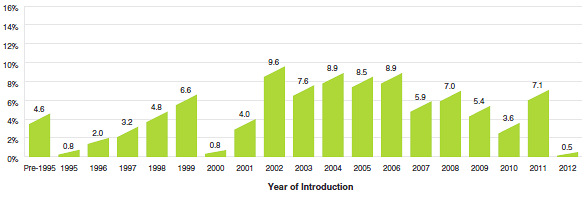
Source: PMPRB
Sales by Therapeutic Class
The PMPRB classifies drug products according to the World Health Organization's (WHO) Anatomical Therapeutic Chemical (ATC) system when it conducts analyses at the level of therapeutic class. This is a hierarchical system that classifies drug products according to their principal therapeutic use and chemical composition. At its first level of aggregation (Level 1), the ATC system classifies drug products according to the element of human anatomy with which they are primarily associated.
Table 9 breaks out sales of patented drug products in Canada in 2012 by major therapeutic class, defined by ATC Level 1. The table gives the 2012 sales for each class, the share of the total sales this represents and the rate at which sales grew relative to 2011. Values in the last column represent the component of overall sales growth attributable to drug products in the corresponding therapeutic class.4 By this measure, antineoplastics and immunomodulating agents made the largest positive contribution to sales growth. This contribution was more than offset by the declining sales of patented drug products related to the cardiovascular system and, secondarily, the blood and blood forming organs.
Table 9 Sales of Patented Drug Products by Major Therapeutic Class, 2012
| Therapeutic class |
2012 sales ($millions) |
Share: 2012 sales (%) |
Growth: 2012/2011 ($millions) |
Growth: 2012/2011 (%) |
Impact on change in expenditure (%) |
| A: Alimentary tract and metabolism |
1,263.7 |
9.8 |
148.5 |
13.3 |
-390.1 |
| B: Blood and blood forming organs |
826.9 |
6.4 |
-126.7 |
-13.3 |
332.8 |
| C: Cardiovascular system |
1,342.8 |
10.5 |
-682.4 |
-33.7 |
1,792.4 |
| D: Dermatologicals |
108.1 |
0.8 |
25.4 |
30.7 |
-66.6 |
| G: Genito-urinary system and sex hormones |
555.8 |
4.3 |
8.0 |
1.5 |
-21.1 |
| H: Systemic hormonal preparations |
55.3 |
0.4 |
-18.2 |
-24.7 |
47.7 |
| J: General antiinfectives for systemic use; and P: Antiparasitic products* |
1,446.5 |
11.3 |
80.9 |
5.9 |
-212.5 |
| L: Antineoplastics and immunomodulating agents |
3,259.4 |
25.4 |
451.7 |
16.1 |
-1,186.5 |
| M: Musculo-skeletal system |
420.4 |
3.3 |
-11.6 |
-2.7 |
30.5 |
| N: Nervous system |
1,941.1 |
15.1 |
134.2 |
7.4 |
-352.3 |
| R: Respiratory system |
1,062.8 |
8.3 |
-101.0 |
-8.7 |
265.4 |
| S: Sensory organs |
505.2 |
3.9 |
57.6 |
12.9 |
-151.2 |
| V: Various |
54.8 |
0.4 |
-4.4 |
-7.5 |
11.6 |
| All therapeutic classes |
12,842.9 |
100.0 |
-38.1 |
-0.3 |
100.0 |
* These groups have been combined for reasons of confidentiality.
Source: PMPRB
End Notes
1 All statistical results for patented drug products reported in this chapter are based on data submitted by patentees as of April 2013. On occasion, patentees report revisions to previously submitted data or provide data not previously submitted. New data of this sort can appreciably affect the statistics in this chapter. To account for this possibility, the PMPRB has adopted the practice of reporting recalculated sales figures (see Trends in Sales of Patented Drug Products), price and quantity indices (see Price Trends and Utilization of Patented Drug Products) and foreign-to-Canadian price ratios (see Comparison of Canadian Prices to Foreign Prices) for the five years preceding the current Annual Report year. All such recalculated values reflect currently available data. Consequently, where data revisions have occurred, values reported here may differ from those presented in earlier Annual Reports.
2 Although based in part on data obtained under license from the MIDAS IMS database, the statements, findings, conclusions, views and opinions expressed in this Annual Report are exclusively those of the PMPRB and are not attributable to IMS AG.
3 Under the scheme applied here, the “exiting drug effect” is the amount of 2012 sales generated by drug products that were under the PMPRB's jurisdiction in 2011 but not in 2012. The “new drug effect” is the amount of 2012 sales generated by drug products that were under the PMPRB's jurisdiction in 2012 but not in 2011. Other effects are derived by means of the relationship:
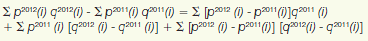
py(i) is the price of drug i in year y, qy(i) is the physical volume of drug i sold in year y and (signifies summation over the set of drug products that were under the PMPRB's jurisdiction in both 2011 and 2012. The left-hand-side of this equation represents the change in total sales of such products between 2011 and 2012. The three terms of the right-hand-side define the volume, price and cross effects, respectively, reported in Table 8.
4 This is obtained as the ratio of the year-over-year change in the dollar value of sales for the therapeutic class in question to the change in sales across all patented drug products.
Price Trends
The PMPRB uses the Patented Medicines Price Index (PMPI) to monitor trends in prices of patented drug products. The PMPI measures the average year-over-year change in the ex-factory prices of patented drug products sold in Canada. The index is constructed using a formula that takes a sales-weighted average of price changes observed at the level of individual drug products.5 This is similar to the approach Statistics Canada uses to construct the Consumer Price Index (CPI). The PMPI is updated every six months using price and sales information submitted by patentees.
It is important to understand the conceptual relationship between the PMPI and drug costs. The PMPI does not measure changes in the utilization of patented drug products; a quantity index, the PMQI, is calculated for this purpose (see Utilization of Patented Drug Products). The PMPI does not measure the cost impact of changes in prescribing patterns or the introduction of new medicines. By design, the PMPI isolates the component of sales growth attributable to changes in prices.
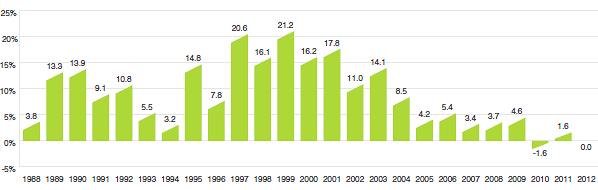
Figure 3 provides year-over-year changes in the PMPI for the years 1988 through 2012. As measured by the PMPI, prices of patented drug products increased slightly, on average, by 0.6% between 2011 and 2012.
Figure 3
Annual Rates of Change, Patented Medicines Price Index (PMPI), 1988–2012
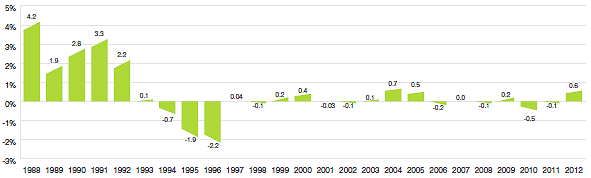
Source: PMPRB
The Patent Act requires the PMPRB to consider changes in the Consumer Price Index (CPI), among other factors, in determining whether the price of a patented drug product is excessive. Figure 4 plots year-over-year rates of change in the PMPI against corresponding changes in the CPI. General price inflation, as measured by the CPI, has exceeded the average increase in patented drug prices almost every year since 1988. In 2012, the CPI rose by 1.5%, while the PMPI on average increased slightly by 0.6%.
Figure 4
Annual Rate of Change, Patented Medicines Price Index (PMPI) and Consumer Price Index (CPI), 1988–2012
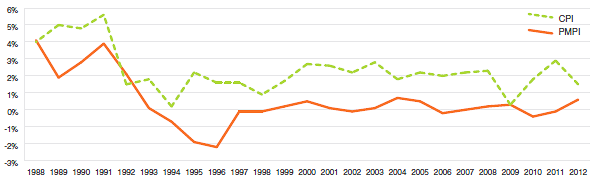
Sources: PMPRB; Statistics Canada
It is not surprising that the PMPI has seldom kept pace with the CPI. The PMPRB's Guidelines allow the price of a patented drug product to rise by no more than the CPI over any three-year period. (The Guidelines also impose a cap on year-over-year price increases equal to one-and-one-half times the current year rate of CPI inflation.) This effectively establishes CPI inflation as an upper bound on the amount by which individual prices may rise over any period of three years.6 Increases in the PMPI normally do not reach this upper bound because many patentees do not raise their prices by the full amount permitted under the Guidelines, or choose to reduce their prices.
Price Change by Therapeutic Class
Table 10 provides average rates of price change among patented drug products at the level of major therapeutic classes. Results in this table were obtained by applying the PMPI methodology to data segregated by their ATC Level I class. The last column provides a decomposition of overall PMPI change, with each entry representing the component of the overall change attributable to drug products in the corresponding therapeutic class. By this measure, slight increase in PMPI (0.6%) reflects a general state of price stability across therapeutic classes. Note that all the therapeutic classes except alimentary tract and metabolism, musculoskeletal system and systemic hormonal preparations saw an average rate of price change below the rate of CPI inflation.7
Table 10 Change in the Patented Medicines Price Index (PMPI), by Major Therapeutic Class, 2012
| Therapeutic class |
Share: 2012 sales (%) |
Price change: 2011 to 2012 (%) |
Contribution: change in PMPI (%) |
| A: Alimentary tract and metabolism |
9.8 |
3.8 |
0.4 |
| B: Blood and blood forming organs |
6.4 |
0.0 |
0.0 |
| C: Cardiovascular system |
10.5 |
0.5 |
0.1 |
| D: Dermatologicals |
0.8 |
0.3 |
0.0 |
| G: Genito-urinary system and sex hormones |
4.3 |
0.9 |
0.0 |
| H: Systemic hormonal preparations |
0.4 |
1.6 |
0.0 |
| J: General Antiinfectives for systemic use; and P: Antiparasitic products* |
11.3 |
-0.7 |
-0.1 |
| L: Antineoplastics and immunomodulating agents |
25.4 |
-0.4 |
-0.1 |
| M: Musculo-skeletal system |
3.3 |
2.0 |
0.1 |
| N: Nervous system |
15.1 |
0.9 |
0.1 |
| R: Respiratory system |
8.3 |
0.8 |
0.1 |
| S: Sensory organs |
3.9 |
0.4 |
0.0 |
| V: Various |
0.4 |
-0.9 |
0.0 |
| All therapeutic classes |
100.0 |
0.6 |
0.6 |
* These groups have been combined for reasons of confidentiality.
Source: PMPRB
Price Change by Class of Customer
Figure 5 presents average rates of price change by class of customer.8 These results were obtained by applying the PMPI methodology separately to sales data for hospital, pharmacy and wholesale customers.9 The 2012 rates of price change for these classes were, respectively, -1.2%, -0.8% and 1.3%.
Figure 5
Annual Rate of Change, Patented Medicines Price Index (PMPI), by Class of Customer, 2009–2012
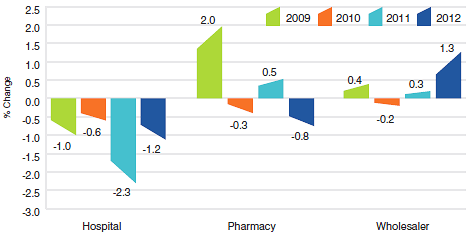
Source: PMPRB
Price Change by Province/Territory
Figure 6 presents average annual rates of price change by province/territory, obtained by applying the PMPI methodology to sales data segregated by the province/territory in which the sale occurred. These results indicate that, between 2011 and 2012, prices of patented drug products in the Yukon fell on average. The largest average price increase occurred in New Brunswick (2.0).
Figure 6
Annual Rate of Price Change, by Province/Territory* and Class of Customer **, 2012
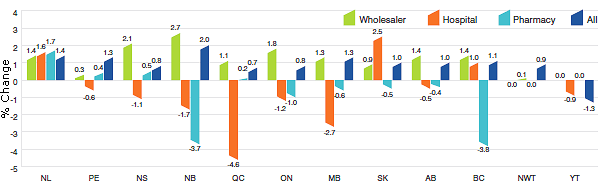
* Values for Nunavut are included in the Northwest Territories (NWT).
** Results for “All” in Figure 6 include the class of customer “other”.
Source: PMPRB
Price Behaviour After Introduction
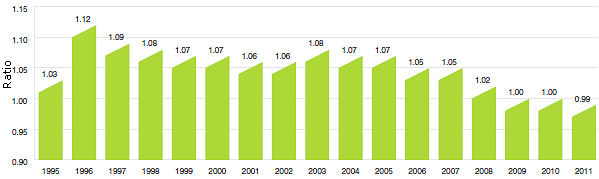
Does the price of a typical patented drug product change much in the years after it enters the Canadian market? To answer this question, Figure 7 provides the average ratio of the 2012 price to introductory price (the price at which the drug product was sold in its first year on the Canadian market).
Figure 7
Average Ratio of 2012 Price to Introductory Price, by Year of Introduction
Source: PMPRB
The results in Figure 7 imply no consistent tendency for prices to either rise or fall after introduction, with the 2012 price of a typical patented drug product being within a few percentage points of its introductory price, regardless of when it was introduced to the Canadian market.10
Price Change by Country
In accordance with the Act and the Regulations, patentees must report publicly available prices of patented drug products for seven foreign comparator countries: France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States.
The PMPRB uses this information to:
- conduct the international price comparison tests specified in its Guidelines
- compare the Canadian prices of patented drug products to those prevailing in other countries
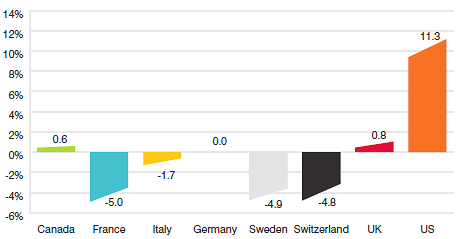
Figure 8 gives the average annual rates of price change for Canada and each of the seven comparator countries. These results were obtained by applying the PMPI methodology (with weights based on Canadian sales patterns) to the international price data that patentees have submitted to the PMPRB. Note that results for the United States are based on prices that incorporate prices from the US Federal Supply Schedule (FSS).11
Figure 8
Annual Average Rates of Price Change, Canada and Comparator Countries, 2012
Source: PMPRB
The results in Figure 8 indicate that in 2012, the United States saw prices rise on average at a rate of 11.3%. United Kingdom saw much more modest average price increases, while prices in France, Italy, Switzerland and Sweden declined.
End Notes
5 These calculations are performed at the level defined by Health Canada's Drug Identification Number (DIN). Each DIN represents a unique combination of active ingredient(s), dosage form, strength(s), brand and manufacturer.
6 It is possible for individual prices (or, for that matter, the PMPI) to rise by more than the CPI in a given year. This can occur when patentees have banked price adjustments in the preceding years. It can also occur when the forecast rate of CPI inflation exceeds the actual rate. To allow patentees to set prices in advance, the CPI-Adjustment Methodology provides for the calculation of the CPI-adjustment factors based on forecast changes in the CPI. This raises the possibility of price increases exceeding CPI inflation whenever forecast CPI inflation exceeds actual CPI inflation. Note that this will not be a permanent gain as the patentee is expected to comply with the actual CPI in all subsequent reporting periods.
7 Suppose R represents the overall rate of change in the PMPI. Suppose there are N therapeutic classes, indexed by 1, 2 … N. Let R(i) represent the average rate of price change in major therapeutic class i obtained by means of the PMPI methodology. Using the fact that R is a sales-weighted average of price changes taken over all patented drug products, it is easy to derive the following relationship:
R = w(1) x R(1) + w(2) x R(2) + … + w(N) x R(N)
where w(i) represents the share of therapeutic class i in the sales of patented drug products. This relationship provides the basis for the decomposition in the last column of Table 10. Each term on its right-hand-side multiplies the average rate of price change for a given therapeutic class by its share of overall sales. The resulting value is readily interpreted as the contribution of the corresponding class to the change in the overall PMPI. Note that the size of this contribution depends on both the rate of price change specific to the class and its relative importance, as measured by its share of sales.
The decomposition in Table 10 is approximate. This is because the weights used to calculate the contribution of each therapeutic class are based on annual sales data, whereas rates of price change (whether overall or by therapeutic class) are calculated from data covering six-month reporting periods. The resulting discrepancy is normally small.
8 Sales of patented drug products are dominated by sales to wholesalers, which accounted for 78.2% of all sales in 2012. Sales to hospitals accounted for another 8.4%, while direct sales to pharmacies accounted for 5.1%. The pharmacy share has fallen precipitously since 2001, when it stood at 20.1%.
9 Results for a fourth class of customer, “Others”, are not provided. This class accounted for about 8.3% of patented drug sales in 2012. Buyers in this class are principally health care institutions other than hospitals, such as clinics and nursing homes. It also includes direct sales to governments. The composition of this class is thought to vary substantially from one year to the next, rendering any analysis of price change in this class of limited value.
10 It must be emphasized that this statement refers to the behaviour of prices on average. There may be instances where individual prices have risen or fallen substantially since introduction.
11 The pharmaceutical industry in the US has argued that the publicly available prices in that country do not reflect actual prices because of confidential discounts and rebates. Effective January 2000, and following public consultation, the PMPRB began including prices listed in the US Federal Supply Schedule (FSS) in calculating the average US price of patented drug products. The FSS prices are negotiated between manufacturers and the US Department of Veterans' Affairs. They are typically less than other publicly available US prices reported to the PMPRB by patentees.
Comparison of Canadian Prices to Foreign Prices
Tables 11 and 12 provide detailed statistics comparing the foreign prices of patented drug products to their Canadian prices. Each table provides two sets of average price ratios. These are differentiated according to the method by which foreign prices were converted to their Canadian dollar equivalents. The tables also give the numbers of drug products (DINs) and the volume of sales encompassed by each reported price ratio.12
The average price ratios given in Tables 11 and 12 are sales-weighted arithmetic means of price ratios obtained for individual drug products, with weights based on Canadian sales patterns. Average price ratios constructed in this way provide exact answers to questions of the type:
How much more/less would Canadians have paid for the patented drug products they purchased in 2012 had they paid Country X prices rather than Canadian prices?
For example, Table 11 states that the 2012 average French-to-Canadian price ratio was 0.76. This means Canadians would have paid 24% less for the patented drug products they purchased in 2012 had they bought these products at French prices.
For many years, the PMPRB has reported average foreign-to- Canadian price ratios with foreign prices converted to their Canadian dollar equivalents by means of market exchange rates. (More exactly, the 36-month moving averages of market rates the PMPRB normally uses in applying its Guidelines.) Table 11 also reports foreign-to-Canadian price ratios with currency conversion at purchasing power parity (PPP). The PPP between any two countries measures their relative costs of living expressed in units of their own currencies. In practice, cost of living is determined by pricing out a standard “basket” of goods and services at the prices prevailing in each country. Because PPPs are designed to represent relative costs of living, they offer a simple way to account for differences in overall national price levels when comparing individual prices, incomes and other monetary values across countries. When applied to the calculation of average foreign-to-Canadian price ratios they produce statistics answering questions of the type:
How much more/less consumption of other goods and services would Canadians have sacrificed for the patented drug products they purchased in 2012 had they lived in Country X?
Questions of this type cannot be answered by simply comparing drug prices. Rather, one must first calculate what each price represents in terms of goods and services foregone. PPPs are designed for such purposes.
Bilateral Comparisons
Table 11 provides bilateral comparisons of prices in each of the PMPRB's seven comparator countries to corresponding Canadian prices. Focusing on the results with currency conversion at market exchange rates, it appears that, as in previous years, Canadian prices were typically within the range of prices observed among the comparator countries. Canadian prices were roughly in line with Swiss prices. Prices in France, Italy, the United Kingdom and Sweden were appreciably lower than Canadian prices, while those in Germany were substantially higher. As in previous years, prices reported for the United States were much higher than prices in Canada or any other comparator country.
Table 11 Average Foreign-to-Canadian Price Ratios, Bilateral Comparisons, 2012
|
Canada |
France |
Italy |
Germany |
Sweden |
Switzerland |
United Kingdom |
United States |
| At Market Exchange Rates |
| Average price ratio 2012 |
1.00 |
0.76 |
0.80 |
1.11 |
0.90 |
1.01 |
0.80 |
2.02 |
| Average price ratio 2011 |
1.00 |
0.84 |
0.84 |
1.20 |
0.95 |
1.03 |
0.82 |
1.98 |
| At Purchasing Power Parities |
| Average price ratio 2012 |
1.00 |
0.79 |
0.91 |
1.24 |
0.84 |
0.82 |
0.89 |
2.42 |
| Average price ratio 2011 |
1.00 |
0.81 |
0.89 |
1.27 |
0.88 |
0.81 |
0.91 |
2.28 |
| Number of patented drug products |
1,264 |
736 |
809 |
903 |
870 |
842 |
864 |
1,070 |
| Sales ($millions) |
12,842.9 |
10,262.5 |
10,389.8 |
11,023.8 |
10,828.0 |
10,768.9 |
10,794.9 |
12,083.6 |
Source: PMPRB
Average price ratios obtained with currency conversion at PPPs tell a somewhat different story. When international differences in cost of living are accounted for, it appears Canadians incurred a substantially larger consumption cost for the patented drug products they purchased in 2012 than did residents of every other comparator country except Germany and the United States.
Figure 9 puts these results in historical perspective. In 2005, Canadian prices were, on average, approximately equal to or below corresponding prices in all comparators other than Italy. By 2012, Canadian prices were decidedly above prices in the United Kingdom, France, and Italy and somewhat higher than prices in Sweden.
Figure 9
Average Foreign-to-Canadian Price Ratios: 2005, 2012
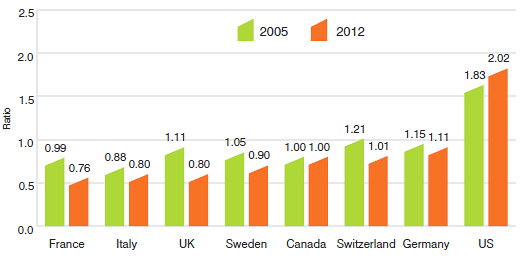
Source: PMPRB
Multilateral Price Comparisons
Table 12 provides average foreign-to-Canadian price ratios using several multilateral measures of foreign prices. The median international price (MIP) is the median of prices observed among the seven comparator countries. Other multilateral price ratios compare the minimum, maximum and simple mean of foreign prices to their Canadian counterparts.
Table 12 Average Foreign-to-Canadian Price Ratios, Multilateral Comparisons, 2012
|
Median |
Minimum |
Maximum |
Mean |
| Average price ratio at market exchange rates |
1.07 |
0.78 |
2.07 |
1.19 |
| Average price ratio at purchasing power parities |
1.09 |
0.84 |
2.44 |
1.29 |
| Number of patented drug products |
1,204 |
1,204 |
1,204 |
1,204 |
| Sales ($millions) |
12,614.0 |
12,614.0 |
12,614.0 |
12,614.0 |
Source: PMPRB
Focusing again on results at market exchange rates, the average MIP-to-Canadian price ratio stood at 1.07 in 2012. (The corresponding value for 2011 was 1.05.) Note that mean foreign prices produce higher foreign-to-Canadian price ratios than do MIPs. This is readily explained by the influence of US prices, which are typically much higher than prices elsewhere. Although US prices nearly always figure importantly in determining mean foreign price, they almost never emerge as median international prices.
Figure 10 puts these results in historical perspective, giving a history of the average MIP-to-Canadian price ratios from 2001 to 2012. Although there has been considerable movement in the ratio over this period, it has remained above parity.
Figure 10
Average Ratio of Median International Price (MIP) to Canadian Price, At Market Exchange Rates, 2001–2012
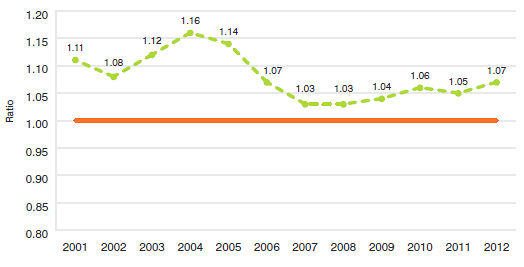
Source: PMPRB
Figure 11 offers more detail on the product-level MIP-to-Canadian ratios underlying the averages reported in Table 12. This figure distributes the 2012 sales of each patented drug product according to the value of its MIP-to-Canadian price ratio (more exactly, according to the range into which the ratio fell).13 These results show substantial dispersion in product-level price ratios: while patented drug products with MIP-to-Canadian price ratios between 0.90 and 1.10 accounted for 26.2% of sales, those with ratios less than 0.90 accounted for 47.4% of sales, and products with ratios exceeding 1.10 accounted for 26.4%.
Figure 11
Range Distribution, Sales, by MIP-to-Canadian Price Ratio, 2012
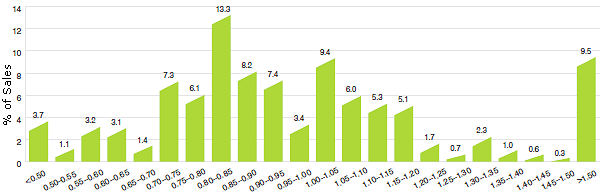
Source: PMPRB
End Notes
12 The number of drug products and sales these ratios encompass vary because it is not always possible to find a matching foreign price for each patented drug product sold in Canada. Note that all of the bilateral average price ratios reported in Table 11 combined represent at least 80% of 2012 Canadian sales, while the multilateral ratios in Table 12 cover over 98%.
13 To produce the results represented in this figure, foreign prices were converted to their Canadian-dollar equivalents at market exchange rates.
Utilization of Patented Drug Products
The price and sales data used to calculate the PMPI also allow the PMPRB to examine trends in the quantities of patented drug products sold in Canada. The PMPRB maintains the Patented Medicines Quantity Index (PMQI) for this purpose. Figure 12 provides average rates of utilization growth, as measured by the PMQI, from 1988 through 2012. These results confirm that in recent years, growth in the utilization of patented drug products has declined significantly, with rates of utilization growth roughly tracking sales growth. This tracking pattern continued in 2012, with the utilization of patented drug products, on average, remaining unchanged (0.0%) between 2011 and 2012 and sales decreasing by 0.3%.
Figure 12
Annual Rate of Change, Patented Medicines Quantity Index (PMQI), 1988–2012
Source: PMPRB
Utilization Growth by Therapeutic Class
Table 13 provides average rates of utilization growth among patented drug products at the level of major therapeutic classes. The results in this table were obtained by applying the PMQI methodology to data segregated by ATC Level I class. As in Table 10, the last column provides an approximate decomposition of overall PMQI change into contributions attributable to each therapeutic class.
In 2012, levels of utilization did not increase in six therapeutic classes. Modest growth in general alimentary tract and metabolism products, antineoplastics and immunomodulating agents, and nervous system products accounted for most of the growth in overall utilization. Drug products in the cardiovascular system and blood and blood forming organs classes declined.
Table 13 Change in the Patented Medicines Quantity Index (PMQI), by Major Therapeutic Class, 2012
| Therapeutic class |
Share:
2012 sales (%) |
Quantity change: 2011 to 2012 (%) |
Contribution: Change in PMQI (%) |
| A: Alimentary tract and metabolism |
9.8 |
20.8 |
2.0 |
| B: Blood and blood forming organs |
6.4 |
-13.8 |
-0.9 |
| C: Cardiovascular system |
10.5 |
-34.7 |
-3.6 |
| D: Dermatologicals |
0.8 |
12.4 |
0.1 |
| G: Genito-urinary system and sex hormones |
4.3 |
3.5 |
0.2 |
| H: Systemic hormonal preparations |
0.4 |
2.9 |
0.0 |
J: General Antiinfectives for systemic use; and
P: Antiparasitic products* |
11.3 |
9.7 |
1.1 |
| L: Antineoplastics and immunomodulating agents |
25.4 |
8.4 |
2.1 |
| M: Musculo-skeletal system |
3.3 |
2.4 |
0.1 |
| N: Nervous system |
15.1 |
11.0 |
1.7 |
| R: Respiratory system |
8.3 |
-2.6 |
-0.2 |
| S: Sensory organs |
3.9 |
12.2 |
0.5 |
| V: Various |
0.4 |
9.9 |
0.0 |
| All therapeutic classes |
100.0 |
0.0 |
0.0 |
* These groups have been combined for reasons of confidentiality.
Source: PMPRB
Canadian Drug Expenditures in the Global Context
IMS Health14 regularly reports on drug sales across a large number of countries. Based on sales data from this source, Figure 13 provides shares of global sales for Canada and each of the seven comparator countries that the PMPRB considers in conducting its price reviews.15 The Canadian market accounted for 2.6% of the global market in 2012.
Figure 13
Distribution of Drug Sales Among Major National Markets, 2012
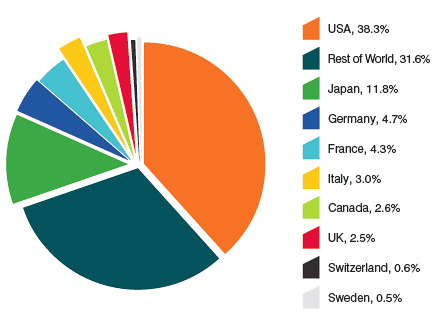
Source: MIDAS©, 2005–2012, IMS Health Incorporated or its affiliates. All rights reserved.16
Figure 14 provides Canada's share of global sales for each of the years 2005 through 2012. The Canadian share has remained between 2.4% and 2.7% throughout this period.
Figure 14
Canada's Share of Drug Sales, 2005–2012
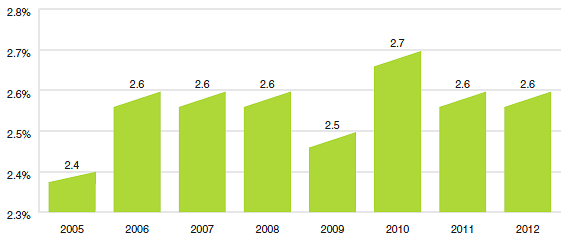
Source: MIDAS©, 2005–2012, IMS Health Incorporated or its affiliates. All rights reserved.16
Figure 15 gives the average annual rate of growth in total drug sales for Canada and the seven comparator countries, individually and collectively. From 2005 to 2012, drug sales in Canada rose at an annual average rate of approximately 4.0%. Drug sales among the seven comparator countries rose at an annual average rate of 3.3% over the same period.
Figure 15
Average Rate of Growth, Drug Sales, at Constant 2012 Market Exchange Rates, by Country, 2005–2012
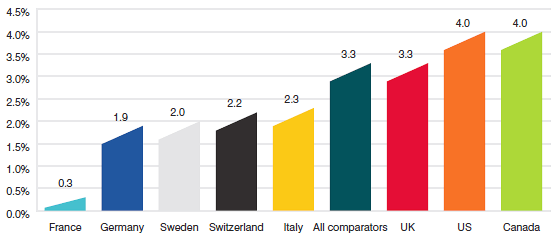
Source: MIDAS©, 2005–2012, IMS Health Incorporated or its affiliates. All rights reserved.16
Figure 16 compares rates of year-over-year growth in drug sales in Canada and the comparator countries combined. In 2012, for the second consecutive year, sales grew at a slower rate in Canada than in the comparator countries.
Figure 16
Average Annual Rate of Change in Drug Sales, at Constant 2012 Market Exchange Rates, Canada and Comparator Countries, 2006–2012
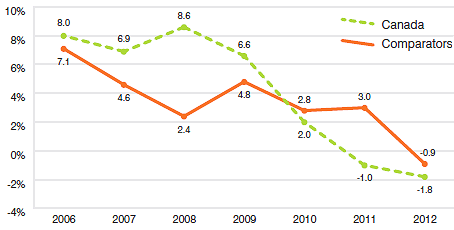
Source: MIDAS©, 2005–2012, IMS Health Incorporated or its affiliates. All rights reserved.16
The proportion of national income allocated to the purchase of drug products provides another way to compare drug costs across countries.17 Figure 17 gives drug expenditures as a share of Gross Domestic Product (GDP) for Canada and the seven comparator countries based on data for 2010. Drug expenditures absorbed between 1.1% and 2.1% of the GDP in the seven comparators. The Canadian value (1.9%) lies near the upper end of this range.
Figure 17
Pharmaceutical Expenditure as a Share of GDP, 2010
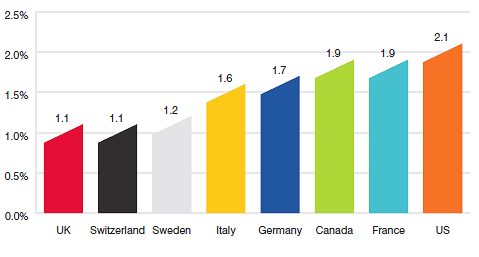
Source: OECD
Table 14 provides historical perspective on the expenditures-to- GDP ratio. Between 2000 and 2010, drug expenditures in Canada grew at approximately twice the rate of GDP growth.
Table 14 Drug Expenditures as a Share of GDP, 2010
|
Share: Drug expenditures/ GDP, 2010 (%) |
Share: Drug expenditures/ GDP, 2000 (%) |
Growth: Drug expenditures, 2000–2010 (%) |
Growth: GDP 2000–2010 (%) |
| Canada |
1.90 |
1.42 |
150.30 |
86.69 |
| France |
1.86 |
1.81 |
74.74 |
70.41 |
| Germany |
1.72 |
1.43 |
97.65 |
64.63 |
| Italy |
1.60 |
1.74 |
65.98 |
80.55 |
| Sweden |
1.21 |
1.18 |
57.35 |
53.50 |
| Switzerland |
1.11 |
1.11 |
58.17 |
58.77 |
| United Kingdom |
1.13 |
1.14 |
51.87 |
52.84 |
| United States |
2.09 |
1.46 |
111.89 |
47.71 |
Source: OECD
Table 15 gives the composition of patentees' sales by therapeutic class for Canada and the seven comparator countries, individually and as an aggregate.18 These results imply a remarkable degree of similarity across countries.
Table 15 Distribution of Drug Sales (%) by Major Therapeutic Class for Canada and Comparator Countries, 2012
| Therapeutic class |
Canada |
Comparators |
France |
Italy |
Germany |
Sweden |
Switzerland |
United Kingdom |
United States |
| A: Alimentary tract and metabolism |
12.6 |
12.2 |
10.6 |
11.2 |
11.5 |
10.1 |
11.1 |
11.0 |
12.6 |
| B: Blood and blood-forming organs |
4.1 |
5.6 |
7.4 |
8.2 |
5.6 |
7.7 |
4.8 |
4.2 |
5.3 |
| C: Cardiovascular system |
14.3 |
10.7 |
12.9 |
13.6 |
9.6 |
6.2 |
12.1 |
8.9 |
10.5 |
| D: Dermatologicals |
3.1 |
2.7 |
2.4 |
2.1 |
2.7 |
2.5 |
3.6 |
3.2 |
2.8 |
| G: Genito-urinary system and sex hormones |
5.2 |
5.0 |
3.3 |
3.9 |
3.8 |
5.0 |
4.6 |
4.7 |
5.4 |
| H: Systemic hormonal preparations |
1.1 |
1.8 |
1.8 |
1.9 |
2.0 |
2.5 |
1.5 |
2.1 |
1.7 |
| J: General antiinfectives for systemic use |
7.3 |
10.6 |
12.2 |
12.9 |
10.1 |
7.8 |
10.7 |
10.4 |
10.4 |
| L: Antineoplastics and immunomodulating agents |
15.6 |
16.7 |
16.7 |
16.6 |
20.6 |
21.4 |
19.8 |
16.4 |
16.1 |
| M: Musculo-skeletal system |
3.7 |
3.1 |
3.4 |
3.9 |
3.7 |
3.0 |
5.0 |
2.6 |
3.0 |
| N: Nervous system |
18.6 |
17.5 |
14.7 |
12.1 |
15.2 |
18.4 |
15.7 |
18.5 |
18.5 |
| P: Antiparasitic products |
0.2 |
0.2 |
0.2 |
0.0 |
0.1 |
0.2 |
0.1 |
0.3 |
0.2 |
| R: Respiratory system |
7.2 |
7.8 |
6.3 |
5.8 |
6.7 |
9.1 |
6.2 |
10.2 |
8.1 |
| S: Sensory organs |
3.1 |
2.5 |
2.9 |
1.9 |
2.6 |
2.6 |
3.1 |
3.3 |
2.4 |
| V: Various |
4.0 |
3.6 |
5.2 |
5.8 |
5.9 |
3.5 |
1.8 |
4.2 |
3.0 |
| All therapeutic classes* |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
100.0 |
* Values in this column may not add to 100.0 due to rounding.
Source: MIDAS©, 2005-2012, IMS Health Incorporated or its affiliates. All rights reserved.16
End Notes
14 Most of the statistical results presented in this section are based on sales data from MIDAS©, 2005–2012, IMS Health Incorporated or its affiliates. All rights reserved.16 These data cover the pharmacy and hospital sectors.
15 The results given in Figures 13 through 16 are based on estimates of ex-factory sales revenues encompassing patented, non-patented branded and generic drug products. These estimates have been converted to Canadian-dollar equivalents at annual average market exchange rates. Fluctuations in these rates can substantially influence these shares.
16 Although based in part on data obtained under license from the MIDAS IMS database, the statements, findings, conclusions, views and opinions expressed in this Annual Report are exclusively those of the PMPRB and are not attributable to IMS AG.
17 Comparisons made on this basis will reflect international differences in prices, overall utilization and patterns of therapeutic choice, as well as differences in national income.
18 Note that the data used to produce Table 15 encompass patented, non-patented branded and generic drug products. Hence, the results reported here for Canada are not directly comparable to those reported in Table 9, which encompass only patented drug products.