Patented Medicine Prices Review Board
The Honourable Ginette Petitpas Taylor
Minister of Health
ISSN 2561-0732
Table of Contents
Chairperson’s message
Results at a glance
Raison d’être, mandate and role: who we are and what we do
Operating context and key risks
Results: what we achieved
Analysis of trends in spending and human resources
Supplementary information
Appendix: definitions
Endnotes
Chairperson’s message
I am pleased to present the Patented Medicine Prices Review Board (PMPRB)’s 2017-18 Departmental Results Report.
The PMPRB is an independent, quasi-judicial administrative agency with a mandate to protect consumers from excessively priced patented medicines and to report to Canadians the latest price trends of all medicines, and on patentees’ research and development spending in Canada.
In 2017-18, the PMPRB’s foremost priority was to continue to advance efforts to modernize its regulatory framework. In December 2017, Health Canada’s proposed amendments to the Patented Medicines Regulations (Regulations) were published in Part 1 of the Canada Gazette. The Regulations are a key deliverable for the Minister of Health in her continuing efforts to improve patient access to necessary prescription medications, including by making them more affordable. If passed, they would require the PMPRB to consider factors beyond simply domestic and international list prices in carrying out its regulatory obligations. During that same month, the PMPRB published a scoping paper, which provided an outline of potential changes to its Guidelines that would operationalize the Regulations and support our objective of moving to a risk-based approach to regulating patented medicine prices.
The PMPRB’s renewed emphasis on consumer-focused regulation made for another busy year of compliance and enforcement activity in 2017-18 with the acceptance of 17 Voluntary Compliance Undertakings (VCUs) and the paying back of excess revenues totalling $4,229,878.65, in addition to price reductions for the affected medicines. In addition to these regulatory reform initiatives, in September, the Alexion matter resulted in the first decision on the merits from a Board panel in an excessive price hearing since 2012.
In terms of its reporting mandate, the PMPRB continued to build strategic partnerships and raise public awareness of its mandate by (1) being more responsive to the specific information needs of pharmaceutical payers while at the same time (2) expanding the scope of its reporting to appeal to a broader stakeholder audience. An example of the former is the National Prescription Drug Utilization Information System’s (NPDUIS) Generics360: Generic Drugs in Canada, 2016 Edition which reports on the latest trends in Canadian generic medicine sales, utilization and pricing within an international context. An example of the latter was the release of the first report in a new three-part series that compares medicine coverage across provincial and federal public drug plans, Alignment among Public Formularies in Canada – Part 1: General Overview. Representatives of the PMPRB also participated in numerous conferences and other public events as speakers, presenters and panelists, and organized information sessions with interested stakeholders to share the results of its analytical studies.
In terms of internal services, in 2017-18 the PMPRB took steps to transform some of its operational support services. In particular, information management and human resource management processes were simplified and administrative burden reduced. Among other benefits, these changes will greatly facilitate the PMPRB’s hiring of the additional personnel it will require to support its new regulatory approach.
We look forward to working with all our stakeholders as the PMPRB transitions toward a modern, risk-based approach to patented medicine price regulation.
Dr. Mitchell Levine
Results at a glance
For more information on the PMPRB’s plans, priorities and results achieved, see the “Results: what we achieved” section of this report.
Priority 1 – Consumer-focused regulation and reporting
The PMPRB’s regulatory mandate is to ensure that the prices of patented medicines sold in Canada are not excessive. By focusing its enforcement resources on cases that have potential precedential value and where payers lack countervailing power, in 2017-18, the Chairperson of the PMPRB accepted 17 Voluntary Compliance Undertakings (VCUs)Footnote i addressing the price of 24 patented medicines. This is the highest number of VCUs in any year over its 30 year history. The PMPRB’s reporting mandate is to provide stakeholders with information on pharmaceutical trends. In 2017-18 the PMPRB published the first report in a three-part series analyzing the gaps and overlaps among provincial and federal public drug plans.
Priority 2 – Framework modernization
In December 2017, following pre-publication of Health Canada’s proposed amendments to the Regulations in Canada Gazette, Part I, the PMPRB published a scoping paper containing a high level overview of how it could operationalize the regulatory amendments through non-binding Guidelines. Consultations on the details of the framework described in the scoping paper will take place over the summer of 2018, with a view to bringing a modern and streamlined regulatory regime into effect in 2019.
Priority 3 – Strategic partnerships and public awareness
The PMPRB continued to build support for its mandate and modernization agenda by participating in countless conferences and stakeholder committees, organizing information sessions with interested stakeholders to share information on its work, and by regularly consulting with the National Prescription Drug Utilization Information System (NPDUIS) Advisory Committee on topics of interest for analytical studies.
Raison d’être, mandate and role: who we are and what we do
Raison d’être
The PMPRB is an independent, quasi-judicial body created by Parliament in 1987. Its mandate is twofold:
- Regulatory – to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and
- Reporting – to report on pharmaceutical trends of all medicines and on research and development (R&D) spending by pharmaceutical patentees.
In carrying out its mandate, the PMPRB ensures that Canadians are protected from excessive prices for patented medicines sold in Canada and that stakeholders are informed on pharmaceutical trends.
Mandate and role
The PMPRB was created as a result of amendments to the Patent Act (Act) in 1987 (Bill C-22), and its remedial powers were supplemented by further amendments in 1993 (Bill C-91). These amendments were part of policy reforms intended to balance consumer protection with measures intended to encourage R&D investment by pharmaceutical patentees.
The PMPRB has a dual mandate:
Regulatory
The PMPRB is responsible for ensuring the factory-gate prices that patentees charge for prescription and non-prescription patented medicines sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, are not excessive. The PMPRB regulates the price of each patented medicine to which Health Canada has assigned a Drug Identification Number (DIN) as part of its price review process. The PMPRB’s mandate also includes medicines that are available under the Special Access Programme, through a Clinical Trial Application, and Investigational New Drug Products. Over-the-counter (OTC) patented medicines and patented medicines for veterinary use are regulated by the PMPRB on a complaints basis.
Reporting
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends of all prescription medicines, and on the R&D expenditures reported by pharmaceutical patentees. In addition, as a result of the establishment of the NPDUISFootnote ii by federal/provincial/territorial (F/P/T) Ministers of Health in September 2001, the PMPRB conducts critical analysis of price, utilization, and cost trends for patented and non-patented prescription medicines to provide Canada’s health system with more comprehensive, accurate information on how all prescription medicines are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on pharmaceutical trends. Increasingly, as part of its reporting function, the PMPRB works closely with provincial and territorial (P/T) governments through NPDUIS, and directly with lead jurisdictions through the Council of the Federation, to provide relevant pricing and market analyses aimed at reducing the prices of prescription medicines purchased by public payers in Canada.
For more general information about the department, see the “Supplementary information” section of this report. For more information on the department’s organizational mandate letter commitments, see the Minister’s mandate letterFootnote iii.
Operating context and key risks
Operating context
Thirty years ago, the PMPRB was created with a mandate to protect consumers by ensuring that the prices charged by patentees for patented medicines sold in Canada are not excessive. Although the PMPRB’s mandate has not changed in the intervening years, many aspects of its operating environment have changed significantly.
Spending on medicines in Canada has increased from less than 6% of total health expenditures, when Medicare was first established, to about 16% in 2018. Medicines are now the second-largest category of spending in health care in Canada, ahead of physician services, with per capita spending on medicines second only to the US. Canada is paying higher prices for prescription medicines than most other developed countries which can result in limited access to innovative medicines, place a financial strain on patients and payers, and mean fewer resources for other critical areas of health care.
Patented medicine prices in Canada are on average approximately 19% higher than the OECD (Organisation for Economic Co-operation and Development) average. This is partly due to the limitations of Canada’s outmoded patented medicine price regulation framework, which assesses Canadian prices against list prices in countries with some of the highest medicine prices in the world (i.e., France, Germany, Italy, Sweden, Switzerland, the UK and the US – known as the “PMPRB7”).
The PMPRB’s price regulation framework is not equipped to address the current and anticipated future pricing challenges, in particular those associated with high-cost specialty medicines. In Budget 2017, the Government announced a substantial increase in funding for the PMPRB as part of the Government’s commitment to making prescription medicines more accessible and affordable for Canadians. In December 2017, Health Canada’s proposed amendments to the Regulations were published in Part 1 of the Canada Gazette. The Regulations are a key deliverable for the Minister of Health in her continuing efforts to improve patient access to necessary prescription medicines, including making them more affordable. Later that month, the PMPRB published a scoping paper which outlines potential changes to its Guidelines that would operationalize the new Regulations and support the PMPRB’s move to a risk-based approach to price regulation that would lower patented medicine prices in Canada.
Key risks
blank
| Risks |
Mitigating strategy and effectiveness |
Link to the department’s Programs |
Link to mandate letter commitments and any government-wide or departmental priorities |
|
Achieving progress on making patented medicines more affordable
There is a risk the modernization of the PMPRB’s regulatory framework will be delayed and the PMPRB will not be able to operationalize the new excessive pricing factors contemplated under the Minister’s proposed amendments to the regulations in the expected timeframe. |
The PMPRB continues to work with Health Canada on its proposed amendments to the Patented Medicines Regulations:
- December 2017, Health Canada’s proposed amendments to the Patented Medicines Regulations were published in Part 1 of the Canada Gazette.
- Later in December, the PMPRB published a scoping paper, which provided an outline of potential changes to its Guidelines which would operationalize the Regulations.
- Summer and early Fall of 2018, the PMPRB will be holding targeted consultations with stakeholders on key technical and operational modalities of the new regime.
- In the event of unforeseen delays in the regulatory change process the PMPRB will assess the situation and take appropriate measures.
|
Patented Medicine Prices Regulation Program |
Minister of Health’s mandate letter commitment: Affordable prescription drugs
PMPRB priority:
Framework modernization
|
|
Acquiring and maintaining infrastructure and hiring personnel for framework modernization
There is a risk that the PMPRB will not be able to attract/retain individuals with skill sets, and expertise, needed and/or have adequate office space to accommodate them, thereby delaying its ability to operationalize the new regulatory framework. |
The PMPRB has:
- established regular HR meetings with managers to proactively address their needs and strengthen HR planning to enable the PMPRB to identify and hire individuals in both the public and private sector with the requisite education, background and experience in a timely fashion.
- developed a detailed plan for accommodations transformation and a monitoring process to ensure that changes and/or delays are identified early and corrective actions initiated to ensure sufficient space to accommodate employees.
|
Patented Medicine Prices Regulations Program
Pharmaceutical Trends Program |
Minister of Health’s mandate letter commitment: Affordable prescription drugs
PMPRB priority:
Framework modernization |
Health Canada is the lead in advancing the proposed amendments to the Regulations. Any delay in the regulatory amendment process will impact the PMPRB’s ability to move forward with changes to its Guidelines, with the result that Canadians’ may not have access to more affordably priced patented medicines for a significant period of time. The PMPRB continues to work closely with Health Canada to provide whatever technical and analytical support is required to ensure the regulatory amendments are finalized on schedule. In the event that the Regulations do not pass in their currently proposed form, or are delayed for an indefinite period of time, the PMPRB will develop contingency plans for moving forward with reform to the Guidelines as per its 2016 discussion paper on Guidelines modernization.
In either event, the PMPRB will require more regulatory officers to deal with increased investigative work that will result from changes to the Guidelines, more legal capacity to manage a greater number of hearings, more expertise in health economics and epidemiology and more modern and user-friendly IT infrastructure. Given some of the skills sets the PMPRB will be seeking are in relatively short supply, there may be delays in completing the staffing processes necessary to bring the requisite new employees onboard. To ensure it has access to the skills needed in the timeframe envisaged, the PMPRB will make use of whatever temporary contracting arrangements are possible with outside experts. Conversely, if the PMPRB is successful in attracting and hiring new staff as per the currently envisaged schedule, it may not have sufficient office space to accommodate them. The PMPRB has developed a detailed plan to address a space shortage which includes the use of flexible work arrangement and the use of temporarily vacant or unused office space.
Results: what we achieved
Programs
Patented Medicine Prices Regulation Program
Description
The PMPRB is an independent quasi-judicial body that is responsible for ensuring that the prices that patentees charge for patented medicines sold in Canada are not excessive based on the price review factors in the Act. To make this determination the Board must consider each of the following factors: prices at which the medicine and other medicines in the same therapeutic class have been sold in Canada and in the seven comparator countries listed in the Patented Medicines Regulations (Regulations); changes in the Consumer Price Index (CPI); and in accordance with the Act, such other factors as may be specified in any regulations made for the purposes of the price review.Footnote iv Under the Act, and as per the Regulations, patentees are required to file price and sales information for each patented medicine sold in Canada, for the duration of the patent(s). Board Staff reviews the introductory and ongoing information filed by patentees, for all patented medicines sold in Canada. When it finds that the price of a patented medicine appears to be excessive, Board Staff will conduct an investigation into the price. An investigation could result in: its closure, where it is concluded that the price was non-excessive; a Voluntary Compliance Undertaking (VCU) by the patentee to reduce the price and offset excess revenues obtained as a result of excessive prices through a payment and/or a price reduction of another patented medicine; or a public hearing to determine if the price is excessive, including any remedial order determined by the Board. In the event that the Board Hearing Panel finds, after a public hearing, that a price is or was excessive, it may order the patentee to reduce the price and take measures to offset any excess revenues. This program, by reviewing the prices charged by patentees for patented medicines sold in Canada, protects Canadians and the health care system from excessive prices.
Results
Results achieved
| Expected results |
Performance indicators |
Target |
Date to achieve target |
2017–18
Actual results |
2016–17
Actual results |
2015–16
Actual results |
| Patentees comply with the Patent Act, the Regulations, and the Excessive Price Guidelines (Guidelines) |
Percentage of patented medicines that are priced within the Guidelines, or at a price which does not trigger the investigation criteria, as a result of voluntary compliance |
95%Footnote 1 |
March 31 of each year |
91.0%Footnote 2 |
92.3%Footnote 3 |
93% |
| Percentage of compliance with Board Orders related to price and/or jurisdiction and with Voluntary Compliance Undertakings (VCUs) |
100% |
March 31 of each year |
100% |
100% |
100% |
| Canadian prices for patented medicines are below the median of international prices |
50%Footnote 4 |
March 31 of each year |
56.4%Footnote 5 |
58% |
n/aFootnote 6 |
Budgetary financial resources (dollars)
2017-18
Main Estimates |
2017-18
Planned spending |
2017–18
Total authorities available for use |
2017–18
Actual spending (authorities used) |
2017–18
Difference (Actual spending minus Planned spending) |
| 6,706,989 |
6,706,989 |
7,189,268 |
5,611,178 |
(1,095,811) |
Human resources (full-time equivalents)
2017-18
Planned full-time equivalents |
2017-18
Actual full-time equivalents |
2017-18
Difference (Actual full-time equivalents minus Planned full-time equivalents) |
| 33.0 |
30.7 |
(2.3) |
The PMPRB’s Compliance Policy was founded on the premise that the most effective and efficient way to protect the public from excessive prices and achieve maximum compliance was through primary reliance on voluntary action by patentees. The PMPRB’s renewed emphasis on consumer-focused regulation has once again resulted in a busy year of compliance and enforcement activity. In 2017-18 the Chairperson accepted 17 VCUs dealing with the prices of 24 patented medicines. These VCUs resulted in price reductions for the affected medicines and the repayment of excess revenues totalling $4,229,878.65.
While in the past the PMPRB enjoyed a high rate of compliance with its Guidelines, in recent years that rate of compliance has been declining and Canadian patented medicine prices have been steadily rising relative to prices in the PMPRB7. It is expected the anticipated changes to operationalize Health Canada’s proposed amendments to the Regulations in order to make patented medicines more affordable for Canadians will initially result in increased incidents of non-compliance as patentees and the PMPRB staff work through the change process. At that time, the PMPRB will review the appropriateness of this indicator and its expected result.
In 2017, Canadian prices were above prices in France, Sweden, Italy and the United Kingdom. Furthermore, as reported in the PMPRB’s 2017 Annual ReportFootnote v, Canadian prices are on average 19% above median OECD prices, which is a slight decrease from 20% in 2016. Canadian prices are third highest among the 31 OECD countries, behind only the United States and Switzerland.
In 2017-18, Health Canada consulted on a proposed comprehensive update to the Regulations to curb excessive medicine prices, ensure long-term sustainability, and align Canada’s medicine pricing policies with like-minded countries. With the comment period for the proposed amendments closed, and in anticipation of final publication of the amendments in Canada Gazette Part II, the PMPRB has resumed its parallel initiative to modernize its Guidelines by consultations on potential changes to its Guidelines. The purpose of these changes is to modernize the PMPRB’s approach to carrying out its mandate to protect Canadians from excessive patented medicine prices. Two main types of changes are contemplated. The first type would operationalize Health Canada’s regulatory amendments intended to make prescription medicines more affordable. The second would allow the PMPRB to make more efficient use of its resources by adopting a risk-based approach to how it regulates prices.
To that end, in June 2018, the PMPRB will hold targeted consultations with stakeholders to seek their feedback on key technical and operational modalities of the new Guidelines that would give effect to these changes. That work will inform the publication of draft guidelines for broader public consultation sometime in the fall. Finalization of the Guidelines will follow final gazetting of the amendments to the Regulations to accommodate the possibility of changes being made between prepublication and final publication that would require adjustments in the PMPRB’s operational approach.
This program has no lower-level programs.
Pharmaceutical Trends Program
Description
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends for all medicines, and R&D expenditures as reported by pharmaceutical patentees. In supporting this requirement, the pharmaceutical trends program provides complete and accurate information on trends in manufacturers' prices of patented medicines sold in Canada and on patentees' research-and-development expenditures to interested stakeholders including: industry (i.e., brand-name, biotech, generic); F/P/T governments; consumer and patient advocacy groups; third party payers; and others. This information also provides assurance to Canadians that the prices of patented medicines are not excessive. In addition, as a result of the establishment of the NPDUIS by F/P/T Ministers of Health, the Federal Minister of Health requested that the PMPRB conduct analysis of price, utilization and cost trends for patented and non-patented prescription medicines so that Canada's health system has more comprehensive, accurate information on how all prescription medicines are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on all prescription medicine prices.
Results
Results achieved
| Expected results |
Performance indicators |
Target |
Date to achieve target |
2017–18
Actual results |
2016–17
Actual results |
2015–16
Actual results |
| Information on pharmaceutical trends and cost drivers is available to stakeholders |
Number of new reports/studies posted on the PMPRB website |
12 reports/ studies |
March 31 of each year |
12 new reports/ studies |
11 new reports/ studies |
15 new reports/ studies |
| Number of presentations made by the PMPRB to an external audience |
10 information sessions |
March 31 of each year |
20 information sessions |
21 information sessions |
25 information sessions |
Budgetary financial resources (dollars)
2017-18
Main Estimates |
2017-18
Planned spending |
2017–18
Total authorities available for use |
2017–18
Actual spending (authorities used) |
2017–18
Difference (Actual spending minus Planned spending) |
| 1,575,179 |
1,575,179 |
1,547,082 |
1,498,746 |
(76,433) |
Human resources (full-time equivalents)
2017-18
Planned full-time equivalents |
2017-18
Actual full-time equivalents |
2017-18
Difference (Actual full-time equivalents minus Planned full-time equivalents) |
| 13.0 |
11.6 |
(1.4) |
In an effort to continue building strategic partnerships and raise public awareness of its consumer protection mandate and expand its reporting scope to appeal to a broader stakeholder audience, in 2017-18, the PMPRB continued its communication activities. This included targeted Twitter campaigns and more conventional (e.g., email and telephone) engagement with domestic, international and specialized media including the CBC, CTV, Radio-Canada, La Presse, The Globe and Mail, Toronto Star, the Canadian Medical Association Journal, Benefits Canada, CBS, Bloomberg News, and Boston Globe among others.
The PMPRB also continued to support and strengthen its NPDUIS engagement activities by regularly consulting with the NPDUIS Advisory Committee, participating in conferences and stakeholder committees, hosting information exchange sessions with researchers, and organizing information sessions with interested stakeholders to share the results of the analytical studies. In 2017-18 the PMPRB released two analytical reports, and seven posters. The first study, Alignment among Public Formularies in Canada, Part 1: General Overview,Footnote vi as well as, the complete list of medicines selected for analysisFootnote vii was published in October 2017 and are available on the Analytical Studies page of the PMPRB website. This study is the first report of a three-part series that analyzes the gaps and overlaps among provincial, territorial and federal public drug listings. In February 2018, the PMPRB released Generics360: Generic Drugs in Canada, 2016 Edition.Footnote viii This report updates previous PMPRB research (PMPRB 2014), highlighting the recent trends in Canadian generic pricing, international price comparisons and market segment analyses.
In addition, the PMPRB published its 2016 Annual ReportFootnote ix and the PMPRB Guidelines Scoping PaperFootnote x.
This program has no lower-level programs.
Internal Services
Description
Internal Services are those groups of related activities and resources that the federal government considers to be services in support of programs and/or required to meet corporate obligations of an organization. Internal Services refers to the activities and resources of the 10 distinct service categories that support Program delivery in the organization, regardless of the Internal Services delivery model in a department. The 10 service categories are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; and Acquisition Services.
Results
Budgetary financial resources (dollars)
2017-18
Main Estimates |
2017-18
Planned spending |
2017–18
Total authorities available for use |
2017–18
Actual spending authorities used) |
2017–18
Difference (Actual spending minus Planned spending) |
| 2,584,153 |
2,584,153 |
2,699,199 |
2,629,270 |
45,117 |
Human resources (full-time equivalents)
2017-18
Planned full-time equivalents |
2017-18
Actual full-time equivalents |
2017-18
Difference (Actual full-time equivalents minus Planned full-time equivalents) |
| 20.0 |
18.0 |
(2.0) |
In 2017-18, the PMPRB secured Public Services and Procurement Canada (PSPC) services to perform digitization of PMPRB collections. By the end of the fiscal year, PSPC had provided a draft business requirements document to facilitate digitization services. The PMPRB also started physical clean-up efforts to divest itself of materials not required and to identify additional material for digitization.
The PMPRB also enhanced its electronic document management system (RIMS) to provide increased user flexibility for document uploading, as well as improved reporting and data extraction functionality. In addition, upgrades were made to the stability of the application and the content type model was simplified to accommodate filing metadata in the SharePoint interface. Work on enhancements to RIMS will continue into 2018-19.
Finally, the PMPRB continued to strengthen its business processes to increase their value to the organization and reduce administrative burden. In particular, the PMPRB made its staffing processes entirely digital, and eliminated duplicative justifications and signing requirements, thereby reducing the administrative burden on its Managers. Regular meetings with managers to proactively address their needs has strengthened Human Resources (HR) planning, making it more valuable to the organization.
Analysis of trends in spending and human resources
Actual expenditures
Departmental spending trend graph
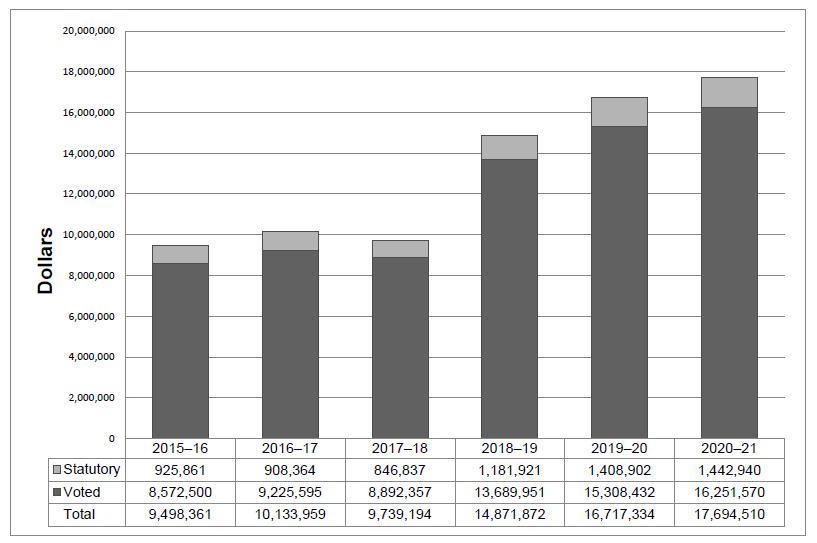
Departmental Spending Trend
The following graph shows the PMPRB's spending trend over time. It illustrates on a bar graph the actual statutory and voted spending for 2015-16 to 2017-18, and planned statutory and voted spending for 2019-20 and 2020-21.
blank
| |
2015-16 |
2016-17 |
2017-18 |
2018-19 |
2019-20 |
2020-21 |
| Statutory |
925,861 |
908,364 |
846,837 |
1,181,921 |
1,408,902 |
1,442,940 |
| Voted |
8,572,500 |
9,225,595 |
8,892,357 |
13,689,951 |
15,308,432 |
16,251,570 |
| Total |
9,498,361 |
10,133,959 |
9,739,194 |
14,871,872 |
16,717,334 |
17,694,510 |
Voted spending in 2017-18 was lower than voted spending in 2016-17 due in large part to decreased spending for hearings, offset by retroactive payments to employees pertaining to collective bargaining. In 2017-18, the PMPRB spent $893,209 from the Special Purpose Allotment (SPA), as compared to $1,883,121 in 2016-17, a difference of $989,912.
As announced in Budget 2017, the PMPRB received additional funding for future years; $3,849,215 in 2018-19, $5,694,677 in 2019-20, $6,671,853 in 2020-21, $7,668,725 in 2021-22 and $5,680,633 in 2022-23 and ongoing, including Employee Benefits Payments (EBP) and increased funding for the SPA.
For purposes of forecasting Planned Spending for 2018-19 and future years it is assumed that the entire SPA funding for hearings will be spent. This is done because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict.
Budgetary performance summary for Programs and Internal Services (dollars)
| Programs and Internal Services |
2017–18
Main Estimates |
2017–18
Planned spending |
2018–19
Planned spending |
2019–20
Planned spending |
2017–18
Total authorities available for use |
2017–18
Actual spending (authorities used) |
2016–17
Actual spending (authorities used) |
2015–16
Actual spending (authorities used) |
| Patented Medicine Prices Regulation Program |
6,706,989 |
a6,706,989 |
9,298,755 |
11,358,172 |
7,189,268 |
5,611,178 |
b6,098,659 |
5,399,127 |
| Pharmaceutical Trends Program |
1,575,179 |
1,575,179 |
1,928,251 |
2,087,393 |
1,547,082 |
1,498,746 |
1,616,278 |
1,688,584 |
| Subtotal |
8,282,168 |
8,282,168 |
11,227,006 |
13,445,565 |
8,736,350 |
7,109,924 |
7,714,937 |
7,087,711 |
| Internal Services |
2,584,153 |
2,584,153 |
3,644,866 |
3,271,769 |
2,699,199 |
2,629,270 |
2,419,022 |
2,410,650 |
| Total |
10,866,321 |
10,866,321 |
c14,871,872 |
16,717,334 |
11,435,549 |
9,739,194 |
10,133,959 |
9,498,361 |
a Actual spending in 2017-18 was lower than actual spending in 2016-17 due in large part to decreased spending for hearings, offset by retroactive payments to employees pertaining to collective bargaining. In 2017-18, the PMPRB spent $893,209 from the Special Purpose Allotment (SPA), as compared to $1,883,121 in 2016-17, a difference of $989,912.
b As announced in Budget 2017, the PMPRB received additional funding for future years: $3,849,215 in 2018-19, $5,694,677 in 2019-20, $6,671,853 in 2020-21, $7,668,725 in 2021-22 and $5,680,633 in 2022-23 and ongoing, including Employee Benefits Payments (EBP) and increased funding for the SPA.
c For purposes of forecasting Planned Spending for 2018-19 and future years it is assumed that the entire SPA funding for hearings will be spent. This is done because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict.
Actual human resources
Human resources summary for Programs and Internal Services
(full-time equivalents)
| Programs and Internal Services |
2015-16
Actual full-time equivalents |
2016-17
Actual full-time equivalents |
2017-18
Planned full-time equivalents |
2017-18
Actual full-time equivalents |
2018-19
Planned full-time equivalents |
2019-20
Planned full-time equivalents |
| Patented Medicine Prices Regulation Program |
31.1 |
31.1 |
33.0 |
30.7 |
38.3 |
46.8 |
| Pharmaceutical Trends Program |
9.2 |
13.3 |
13.0 |
11.6 |
12.7 |
13.7 |
| Subtotal |
33.9 |
44.4 |
46.0 |
42.3 |
51.0 |
60.5 |
| Internal Services |
22.3 |
19.3 |
20.0 |
18.0 |
21.0 |
21.5 |
| Total |
56.2 |
63.7 |
66.0 |
60.3 |
a72.0 |
82.0 |
a As announced in Budget 2017, the PMPRB received additional funding for future years; some of that funding will be used to staff additional FTEs.
Expenditures by vote
For information on the PMPRB’s organizational voted and statutory expenditures, consult the Public Accounts of Canada 2017-2018.Footnote xi
Government of Canada spending and activities
Information on the alignment of the PMPRB’s spending with the Government of Canada’s spending and activities is available in the GC InfoBase.Footnote xii
Financial statements and financial statements highlights
Financial statements
The PMPRB’s financial statements (unaudited) for the year ended March 31, 2018, are available on the departmental websiteFootnote xiii.
Financial statements highlights
Condensed Statement of Operations (unaudited) for the year ended March 31, 2018 (dollars)
| Financial information |
2017–18
Planned
results |
2017–18
Actual results |
2016–17
Actual results |
Difference
(2017–18
Actual results
minus
2017–18
Planned results) |
Difference
(2017–18
Actual results
minus
2016–17
Actual results) |
| Total expenses |
11,991,436 |
11,175,045 |
11,140,340 |
(816,391) |
34,705 |
| Total revenues |
- |
686 |
9,297 |
686 |
(8,611) |
| Net cost of operations before government funding and transfers |
11,991,436 |
11,174,359 |
11,131,043 |
(817,077) |
43,316 |
Condensed Statement of Financial Position (unaudited) as at March 31, 2018 (dollars)
| Financial Information |
2017–18 |
2016–17 |
Difference
(2017–18
minus
2016–17) |
| Total net liabilities |
2,087,459 |
2,294,983 |
(207,524) |
| Total net financial assets |
1,307,889 |
1,722,674 |
(414,785) |
| Departmental net debt |
779,570 |
572,309 |
207,261 |
| Total non-financial assets |
120,272 |
100,895 |
19,377 |
| Departmental net financial position |
(659,298) |
(471,414) |
(187,884) |
Supplementary Information
Corporate information
Organizational profile
Appropriate minister: The Honourable Ginette Petitpas Taylor
Institutional head: Dr. Mitchell Levine, Chairperson
Ministerial portfolio: Health
Enabling instrument(s): Patent Act Footnote xiv and Patented Medicines Regulations Footnote xv
Year of incorporation / commencement: 1987
Other:The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act (Act) set out in sections 79 to 103. Although the Patented Medicine Prices Review Board (PMPRB) is part of the Health Portfolio, because of its quasi-judicial responsibilities the PMPRB carries out its mandate at arm’s length from the Minister. It also operates independently of Health Canada, which approves medicines for safety, efficacy and quality; other Health Portfolio members, such as the Public Health Agency of Canada, the Canadian Institutes of Health Research and the Canadian Food Inspection Agency; and federal, provincial and territorial (F/P/T) public drug plans, which approve the listing of medicines for their respective formularies for reimbursement purposes; and the Common Drug Review, administered by the Canadian Agency for Drugs and Technologies in Health (CADTH), which recommends medicines that should qualify for reimbursement purposes by participating public drug plans.
Reporting framework
The PMPRB’s Strategic Outcome and Program Alignment Architecture of record for 2017–18 are shown below.
1. Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
1.1 Program: Patented Medicine Prices Regulation Program
1.2 Program: Pharmaceutical Trends Program
Internal Services
Supporting information on lower-level programs
The PMPRB does not have any lower-level programs. The PMPRB only has one strategic outcome, two supporting programs and internal services.
Supplementary information tables
The following supplementary information tables are available on the PMPRB’s website:
Federal tax expenditures
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax Expenditures.Footnote xvii This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs. The tax measures presented in this report are the responsibility of the Minister of Finance.
Organizational contact information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 288-9635
Toll-free no.: 1-877-861-2350
Facsimile: (613) 288-9643
TTY: (613) 288-9654
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Appendix: definitions
appropriation (crédit)
Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
budgetary expenditures (dépenses budgétaires)
Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
Departmental Plan (plan ministériel)
A report on the plans and expected performance of an appropriated department over a three-year period. Departmental Plans are tabled in Parliament each spring.
Departmental Results Report (rapport sur les résultats ministériels)
A report on an appropriated department’s actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
evaluation (évaluation)
In the Government of Canada, the systematic and neutral collection and analysis of evidence to judge merit, worth or value. Evaluation informs decision making, improvements, innovation and accountability. Evaluations typically focus on programs, policies and priorities and examine questions related to relevance, effectiveness and efficiency. Depending on user needs, however, evaluations can also examine other units, themes and issues, including alternatives to existing interventions. Evaluations generally employ social science research methods.
experimentation (expérimentation)
Activities that seek to explore, test and compare the effects and impacts of policies, interventions and approaches, to inform evidence-based decision-making, by learning what works and what does not.
full-time equivalent (équivalent temps plein)
A measure of the extent to which an employee represents a full person-year charge against a departmental budget. Full-time equivalents are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
gender-based analysis plus (GBA+) (analyse comparative entre les sexes plus [ACS+])
An analytical approach used to assess how diverse groups of women, men and gender-diverse people may experience policies, programs and initiatives. The “plus” in GBA+ acknowledges that the gender-based analysis goes beyond biological (sex) and socio-cultural (gender) differences. We all have multiple identity factors that intersect to make us who we are; GBA+ considers many other identity factors, such as race, ethnicity, religion, age, and mental or physical disability. Examples of GBA+ processes include using data disaggregated by sex, gender and other intersecting identity factors in performance analysis, and identifying any impacts of the program on diverse groups of people, with a view to adjusting these initiatives to make them more inclusive.
government-wide priorities (priorités pangouvernementales)
For the purpose of the 2017–18 Departmental Results Report, those high-level themes outlining the government’s agenda in the 2015 Speech from the Throne, namely: Growth for the Middle Class; Open and Transparent Government; A Clean Environment and a Strong Economy; Diversity is Canada’s Strength; and Security and Opportunity.
horizontal initiative (initiative horizontale)
An initiative where two or more departments are given funding to pursue a shared outcome, often linked to a government priority.
Management, Resources and Results Structure (structure de gestion, des ressources et des résultats)
A comprehensive framework that consists of an organization’s inventory of programs, resources, results, performance indicators and governance information. Programs and results are depicted in their hierarchical relationship to each other and to the Strategic Outcome(s) to which they contribute. The Management, Resources and Results Structure is developed from the Program Alignment Architecture.
non-budgetary expenditures (dépenses non budgétaires)
Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
performance (rendement)
What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
performance indicator (indicateur de rendement)
A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
performance reporting (production de rapports sur le rendement)
The process of communicating evidence-based performance information. Performance reporting supports decision making, accountability and transparency.
plan (plan)
The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
planned spending (dépenses prévues)
For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts that receive Treasury Board approval by February 1. Therefore, planned spending may include amounts incremental to planned expenditures presented in the Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
priority (priorité)
A plan or project that an organization has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired Strategic Outcome(s) or Departmental Results.
program (programme)
A group of related resource inputs and activities that are managed to meet specific needs and to achieve intended results and that are treated as a budgetary unit.
Program Alignment Architecture (architecture d’alignement des programmes)
A structured inventory of an organization’s programs depicting the hierarchical relationship between programs and the Strategic Outcome(s) to which they contribute.
result (résultat)
An external consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization’s influence.
statutory expenditures (dépenses législatives)
Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
Strategic Outcome (résultat stratégique)
A long-term and enduring benefit to Canadians that is linked to the organization’s mandate, vision and core functions.
sunset program (programme temporisé)
A time-limited program that does not have an ongoing funding and policy authority. When the program is set to expire, a decision must be made whether to continue the program. In the case of a renewal, the decision specifies the scope, funding level and duration.
target (cible)
A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
voted expenditures (dépenses votées)
Expenditures that Parliament approves annually through an Appropriation Act. The Vote wording becomes the governing conditions under which these expenditures may be made.