Alignment of 2016–17 Planned Spending With the Whole-of-Government FrameworkFootnote x (dollars)
| Strategic Outcome |
Program |
Spending Area |
Government of Canada Outcome |
2016-17 Planned Spending |
| Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
|
Patented Medicine Prices Regulation Program |
Social affairs |
Healthy Canadians |
6,646,758 |
| Pharmaceutical Trends Program |
Social affairs |
Healthy Canadians |
1,704,508 |
Total Spending by Spending Area (dollars)
| Spending Area |
Total Planned Spending |
| Economic affairs |
|
| Social affairs |
8,351,266 |
| International affairs |
|
| Government affairs |
|
Departmental Spending Trend
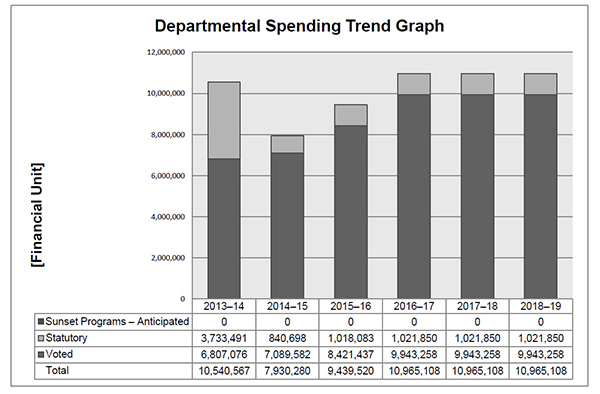
Departmental Spending Trend
The graph shows the Patented Medicine Prices Review Board's planned and actual spending trend over time. It illustrates on a bar graph the actual statutory and voted spending in 2013-14, 2014-15, and planned statutory and voted spending in 2015-16, 2016-17, 2017-18 and 2018-19.
The variance between Statutory Expenditures for 2013–14 and 2014–15 is largely due to additional funding received through an adjustment warrant to cover the amount ordered by the Federal Court to refund to a patentee. The Federal Court quashed a Board Order and directed in its judgement that a payment of excess revenues in the sum of $2,801,285 be returned by the PMPRB to the patentee with appropriate interest and specified costs.
The 2015–16 Main Estimates amount includes funding for a Special Purpose Allotment (SPA) in the amount of $2,470,000. The SPA is for conducting Public Hearings and can only be used to cover costs such as external legal counsel, expert witnesses, etc. Any unspent SPA funds are returned to the Consolidated Revenue Fund (CRF).
Due to challenges related to forecasting the number and complexity of hearings, for purposes of forecasting Planned Spending for 2016–17 and future years it is assumed that the entire SPA funding will be spent.
Estimates by Vote
For information on the PMPRB’s organizational appropriations, consult the 2016–17 Main Estimates.Footnote xi
Section II: Analysis of Programs by Strategic Outcome
Strategic Outcome:
Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
Program 1.1: Patented Medicine Prices Regulation Program
Description
The PMPRB is an independent quasi-judicial body that is responsible for ensuring that the prices that patentees charge for patented medicines sold in Canada are not excessive based on the price review factors in the Patent Act (Act). To make this determination the Board must consider each of the following factors: prices at which the medicine and other medicines in the same therapeutic class have been sold in Canada and in the seven comparator countries listed in the Patented Medicines Regulations (Regulations); changes in the Consumer Price Index (CPI); and in accordance with the Act, such other factors as may be specified in any regulations made for the purposes of the price review.Footnote xii Under the Act, and as per the Regulations, patentees are required to file price and sales information for each patented medicine sold in Canada, for the duration of the patent(s). Board Staff reviews the introductory and ongoing information filed by patentees, for all patented medicines sold in Canada. When it finds that the price of a patented medicine appears to be excessive, Board Staff will conduct an investigation into the price. An investigation could result in: its closure, where it is concluded that the price was non-excessive; a Voluntary Compliance Undertaking (VCU) by the patentee to reduce the price and offset excess revenues obtained as a result of excessive prices through a payment and/or a price reduction of another patented drug product; or a public hearing to determine if the price is excessive, including any remedial order determined by the Board. In the event that the Board Hearing Panel finds, after a public hearing, that a price is or was excessive, it may order the patentee to reduce the price and take measures to offset any excess revenues. This program, by reviewing the prices charged by patentees for patented medicines sold in Canada, protects Canadians and the health care system from excessive prices.
Budgetary Financial Resources (dollars)
2016–17
Main Estimates |
2016–17
Planned Spending |
2017–18
Planned Spending |
2018–19
Planned Spending |
| 6,646,758 |
6,646,758 |
6,646,758 |
6,646,758 |
Human Resources (Full-Time Equivalents [FTEs])
| 2016–17 |
2017–18 |
2018–19 |
| 40 |
40 |
40 |
Performance Measurement
| Expected Results |
Performance Indicators |
Targets |
Date to Be Achieved |
| Patentees comply with the Patent Act, the Regulations, and the Excessive Price Guidelines (Guidelines) |
Percentage of patented medicines that are priced, as a result of voluntary compliance, within the Guidelines or at a price which does not trigger the investigation criteria |
95% |
March 31 of each year |
| Percentage of compliance with Board Orders related to price and/or jurisdiction and with Voluntary Compliance Undertakings (VCUs) |
100% |
March 31 of each year |
| Canadian prices for patented medicines are on average in line with prices in the seven comparator countries listed in the Regulations |
Canadian prices for patented medicines are below the median of international prices |
50% |
March 31 of each year |
Planning Highlights
The PMPRB will focus its enforcement resources on cases that are most relevant to payers, and that will raise issues which could clarify certain aspects of the PMPRB’s regulatory framework and make it a more effective consumer champion. The PMPRB will also consult on whether and to what extent changes to consumer protection powers are warranted to ensure Canadian patented drug costs remain affordable.
Program 1.2: Pharmaceutical Trends Program
Description
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends for all drugs, and R&D expenditures as reported by pharmaceutical patentees. In supporting this requirement, the pharmaceutical trends program provides complete and accurate information on trends in manufacturers' prices of patented medicines sold in Canada and on patentees' research-and-development expenditures to interested stakeholders including: industry (i.e., brand-name, biotech, generic); F/P/T governments; consumer and patient advocacy groups; third party payers; and others. This information also provides assurance to Canadians that the prices of patented medicines are not excessive. In addition, as a result of the establishment of the NPDUIS by F/P/T Ministers of Health the Federal Minister of Health requested that the PMPRB conduct analysis of price, utilization and cost trends for patented and non-patented prescription drugs so that Canada's health system has more comprehensive, accurate information on how all prescription drugs are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on all prescription drug prices.
Budgetary Financial Resources (dollars)
2016–17
Main Estimates |
2016–17
Planned Spending |
2017–18
Planned Spending |
2018–19
Planned Spending |
| 1,704,508 |
1,704,508 |
1,704,508 |
1,704,508 |
Human Resources (Full-Time Equivalents [FTEs])
| 2016–17 |
2017–18 |
2018–19 |
| 12 |
12 |
12 |
Performance Measurement
| Expected Results |
Performance Indicators |
Targets |
Date to Be Achieved |
| Information on pharmaceutical trends and cost drivers is available to stakeholders |
Number of new reports/studies posted on the PMPRB website |
12 reports/studies |
March 31 each year |
| Number of presentations made by the PMPRB to an external audience |
10 information sessions |
March 31 each year |
Planning Highlights
The PMPRB will adopt a more proactive approach to communicating its regulatory and reporting achievements to the public and will build on its reputation as an honest broker in identifying, analyzing and reporting on pharmaceutical issues. This includes intensifying its partnership with public payers to better anticipate their market intelligence requirements and specific information needed in the context of pCPA negotiations, and expanding the scope of pharmaceutical topics on which it reports to provide private payers and consumers with information to help them make better, more cost effective choices. The PMPRB will also strengthen ties with pricing and reimbursement authorities in other countries in order to share market intelligence and stay abreast of the latest developments in this area. As in past years, the PMPRB will publish its NPDUIS Research Agenda which reflects the priorities identified by the NPDUIS Advisory Committee and lists the reports anticipated for completion and publication each year.
Internal Services
Description
Internal Services are groups of related activities and resources that are administered to support the needs of programs and other corporate obligations of an organization. Internal services include only those activities and resources that apply across an organization, and not those provided to a specific program. The groups of activities are Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; and Acquisition Services.
Budgetary Financial Resources (dollars)
2016–17
Main Estimates |
2016–17
Planned Spending |
2017–18
Planned Spending |
2018–19
Planned Spending |
| 2,613,842 |
2,613,842 |
2,613,842 |
2,613,842 |
Human Resources (FTEs)
| 2016–17 |
2017–18 |
2018–19 |
| 19 |
19 |
19 |
Planning Highlights
As a micro agency, the PMPRB boasts a small but agile workforce with a diverse range of skill sets and professional backgrounds. To maintain standards of excellence and convince employees that the PMPRB is a desirable organization in which to build a career, the PMPRB will continue to inform and engage employees in the strategic planning process, and provide them clear direction on work objectives and expected behaviours in order to promote a culture of consistent high performance. It will implement a comprehensive internal communications strategy to enable more structured dialogue between branches, management and employees and it will put systems in place to enable employees to rate their managers on the degree to which they are meeting their engagement objectives. The PMPRB will also provide access to a wide range of learning and developmental opportunities, including, but not limited to, mentoring and developmental assignments.
Section III: Supplementary Information
Future-Oriented Condensed Statement of Operations
The Future-Oriented Condensed Statement of Operations provides a general overview of the PMPRB’s operations. The forecast of financial information on expenses and revenues is prepared on an accrual accounting basis to strengthen accountability and to improve transparency and financial management.
Because the Future-Oriented Condensed Statement of Operations is prepared on an accrual accounting basis, and the forecast and planned spending amounts presented in other sections of the Report on Plans and Priorities are prepared on an expenditure basis, amounts may differ.
A more detailed Future-Oriented Statement of Operations and associated notesFootnote xiii, including a reconciliation of the net cost of operations to the requested authorities, are available on the PMPRB’s website.
Future-Oriented Condensed Statement of Operations
For the Year Ended March 31, 2016
(dollars)
| Financial Information |
2015–16 Forecast results |
2016–17 Planned Results |
Difference
(2016–17 Planned Results minus 2015–16 Forecast Results)
|
| Total expenses |
10,563,636 |
12,157,399 |
1,593,763 |
| Total revenuesFootnote1 |
48 |
- |
(48) |
| Net cost of operations before government funding and transfers |
10,563,588 |
12,157,399 |
1,593,811 |
PMPRB is projecting $12.2M in expenses based on 2016-17 Main Estimates and accrued information. This amount does not include future supplementary estimates. It represents an increase of $1.6 M from 2015-16 projections.
This increase is primarily attributable to:
- Planned spending in 2016–17 is based on the assumption that the PMPRB will spend the full $2.47 million held in the SPA reserved for conducting public hearings. This is done because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict.
Supplementary Information Tables
The supplementary information tables listed in the 2016–17 Report on Plans and Priorities are available on the PMPRB’s website.
Tax Expenditures and Evaluations
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Tax Expenditures and EvaluationsFootnote xvi publication. The tax measures presented in that publication are the responsibility of the Minister of Finance.
Section IV: Organizational Contact Information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Toll-free no.: 1-877-861-2350
Facsimile: (613) 952-7626
TTY: (613) 957-4373
Email: pmprb@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Appendix: Definitions
Appropriation: Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
budgetary expenditures: Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
Departmental Performance Report: Reports on an appropriated organization’s actual accomplishments against the plans, priorities and expected results set out in the corresponding Reports on Plans and Priorities. These reports are tabled in Parliament in the fall.
full-time equivalent: A measure of the extent to which an employee represents a full person-year charge against a departmental budget. Full-time equivalents are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
Government of Canada outcomes: A set of 16 high-level objectives defined for the government as a whole, grouped in four spending areas: economic affairs, social affairs, international affairs and government affairs.
Management, Resources and Results Structure: A comprehensive framework that consists of an organization’s inventory of programs, resources, results, performance indicators and governance information. Programs and results are depicted in their hierarchical relationship to each other and to the Strategic Outcome(s) to which they contribute. The Management, Resources and Results Structure is developed from the Program Alignment Architecture.
non-budgetary expenditures: Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
performance: What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
performance indicator: A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
performance reporting: The process of communicating evidence-based performance information. Performance reporting supports decision making, accountability and transparency.
planned spending: For Reports on Plans and Priorities (RPPs) and Departmental Performance Reports (DPRs), planned spending refers to those amounts that receive Treasury Board approval by February 1. Therefore, planned spending may include amounts incremental to planned expenditures presented in the Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their RPPs and DPRs.
plans: The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
priorities: Plans or projects that an organization has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired Strategic Outcome(s).
program: A group of related resource inputs and activities that are managed to meet specific needs and to achieve intended results and that are treated as a budgetary unit.
Program Alignment Architecture: A structured inventory of an organization’s programs depicting the hierarchical relationship between programs and the Strategic Outcome(s) to which they contribute.
Report on Plans and Priorities: Provides information on the plans and expected performance of appropriated organizations over a three-year period. These reports are tabled in Parliament each spring.
results: An external consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization’s influence.
statutory expenditures: Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
Strategic Outcome: A long-term and enduring benefit to Canadians that is linked to the organization’s mandate, vision and core functions.
sunset program: A time-limited program that does not have an ongoing funding and policy authority. When the program is set to expire, a decision must be made whether to continue the program. In the case of a renewal, the decision specifies the scope, funding level and duration.
target: A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
voted expenditures: Expenditures that Parliament approves annually through an Appropriation Act. The Vote wording becomes the governing conditions under which these expenditures may be made.
whole-of-government framework: Maps the financial contributions of federal organizations receiving appropriations by aligning their Programs to a set of 16 government-wide, high-level outcome areas, grouped under four spending areas.