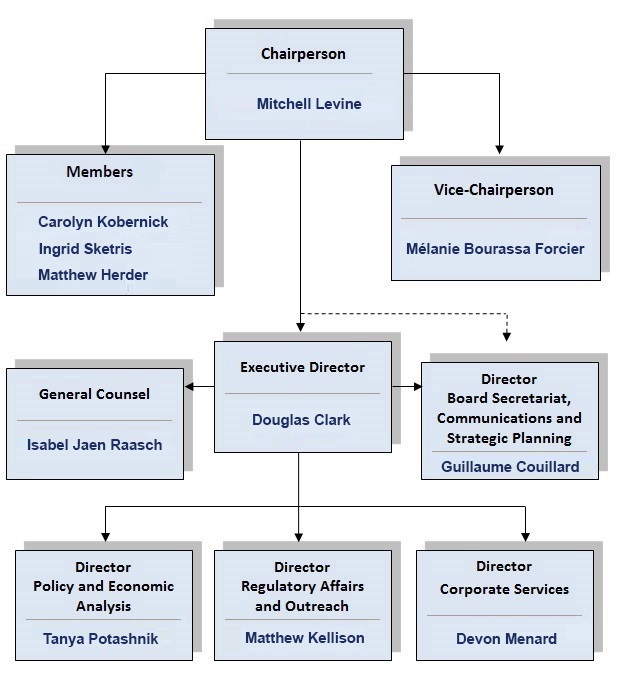
Board Members
The Board consists of not more than five members who serve on a part-time basis, appointed by the Governor-in-Council, including a Chairperson and a Vice-chairperson. The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB with the authority and responsibility to supervise and direct its work. By operation of law, the Vice-chairperson exercises all the powers and functions of the Chairperson should the Chairperson be absent or incapacitated, or if the office of the Chairperson is vacant.
Find out more about the roles and responsibilities of Board Members.
Chairperson
Mitchell Levine
Dr. Mitchell Levine, MD, MSc, FRCPC, FACP, FISPE was appointed Chairperson of the Board on February 13, 2018. He has served as a Member and Vice-Chairperson of the Board since 2011.
Dr. Levine is a Professor at McMaster University in Hamilton in the Department of Health Research Methods, Evidence and Impact and in the Department of Medicine, Division of Clinical Pharmacology and Toxicology. He is also an Assistant Dean in the Faculty of Health Sciences and a faculty member of the Centre for Health Economics and Policy Analysis at McMaster.
Dr. Levine received his MD degree from the University of Calgary, which was followed by postgraduate training in Internal Medicine and Clinical Pharmacology at the University of Toronto. He received an MSc degree in Clinical Epidemiology from McMaster University.
Dr. Levine is a consultant physician in the fields of internal medicine and clinical pharmacology in Hamilton. On an ad hoc basis, he acts as a clinical pharmacology consultant to the Ontario Ministry of Health and Long-Term Care. Prior to his appointment to the Board, Dr. Levine was a member of the Patented Medicine Prices Review Board's Human Drug Advisory Panel.
Vice-Chairperson
Mélanie Bourassa Forcier
Mélanie Bourassa Forcier is an Associate Professor in the Faculty of Law at the Université de Sherbrooke. She directs the Law and Health Policy, and Law and Life Sciences programs. She has expertise in the regulation, marketing and reimbursement of new medical technologies.
Professor Bourassa Forcier has published numerous books and articles on the subject of pharmaceutical regulation and health law. She holds a Ph.D. in Pharmaceutical Patent Law from McGill University, an MSc in International Health Policy from the London School of Economics and Political Science, and an LL.L. from the University of Ottawa.
Members
Carolyn Kobernick
Carolyn Kobernick is a lawyer and former career public servant. Prior to her retirement in 2013, Ms. Kobernick had been Assistant Deputy Minister of Public Law for the Department of Justice since 2006. As principal counsel to the Minister of Justice and Attorney General of Canada, Ms. Kobernick was instrumental in the development and delivery of policy for the Public Law sector. In addition to identifying key strategic, legal and operational matters, she tackled cross-cutting national issues as the liaison between the Department of Justice and other government organizations.
Ms. Kobernick joined the Department of Justice in 1980 where she practiced litigation and tax law at the Toronto Regional office. In 1991 she was appointed Senior General Counsel, Deputy Head, Business and Regulatory Law Portfolio, after working for over a decade in the legal services unit of the Correctional Service of Canada. In her role as Senior General Counsel, Ms. Kobernick was involved in complex policy and operational issues affecting the Government of Canada, including the Alaska Pipeline and Mackenzie Valley Pipeline files and the Sponsorship file.
During her career with the public service, Ms. Kobernick actively participated in many high-profile initiatives. She was Chair of the National Legal Advisory Committee and Departmental Champion for Aboriginal People and Gender Equity. She also served as the Senior Department of Justice official at the Domestic Affairs Cabinet Committee, and was appointed Senior Legal Advisor to the Government of Canada for the 2004 Gomery Inquiry.
Ms. Kobernick holds a B.C.L. and L.L.B. from McGill University and is a member of the bar of Ontario. In 2012 she obtained a Certificate in Adjudication for Administrative Agencies Boards and Tribunals from the Osgoode Hall Law School and The Society of Adjudicators and Regulators.
Dr. Ingrid Sketris
Dr. Ingrid Sketris is a licensed pharmacist and a professor at the College of Pharmacy, Dalhousie University, with cross appointments to Medicine and Health Administration.
Dr. Sketris received her Doctor of Pharmacy in 1979 from the University of Minnesota, followed by her residency in Clinical Toxicology at the University of Tennessee Centre for the Health Sciences. She also received a Masters in Public Administration/Health Services Administration from Dalhousie University.
She is a leader in pharmacy, and has served as President of the Association of Faculties of Pharmacy of Canada and as a board member of the Canadian Council for Accreditation of Pharmacy Programs.
Dr. Sketris is a Fellow of the Canadian Society of Hospital Pharmacists, the American College of Clinical Pharmacy and the Canadian Academy of Health Sciences. She was previously elected to the US National Academies of Practice.
Matthew Herder
Matthew Herder is the Director of the Health Law Institute at Dalhousie University as well as an Associate Professor in the Department of Pharmacology in the Faculty of Medicine, with a cross-appointment to the Schulich School of Law.
Herder’s research focuses on biomedical innovation policy, with a particular emphasis on intellectual property rights and the regulation of biopharmaceutical interventions. His work is often interdisciplinary and policy-oriented, and he has received grants from the Canadian Institutes of Health Research and the Royal Society of Canada, in addition to appearing as an expert witness before several Parliamentary committees on pharmaceutical regulation and policy.
Prior to arriving at Dalhousie, Herder was the Ewing Marion Kauffman Foundation Legal Research Fellow at New York University’s School of Law. He was a Law Clerk at the Federal Court of Canada and was admitted to the Law Society of Upper Canada. Herder holds a Master of the Science of Law degree from Stanford Law School as well as two law degrees from Dalhousie University.
Directorates
Regulatory Affairs and Outreach
The Regulatory Affairs and Outreach Branch reviews the prices of patented drug products sold in Canada to ensure that they are not excessive; encourages patentees to comply voluntarily with the Board’s Guidelines; implements related compliance policies; and investigates complaints into the prices of patented medicines. This branch also informs and educates patentees on the Board’s Guidelines and filing requirements.
Policy and Economic Analysis
The Policy and Economic Analysis Branch develops policy and strategic advice; makes recommendations on possible amendments to the Board’s Guidelines; conducts research and analysis on the prices of drugs, pharmaceutical market developments and R&D trends; and publishes studies aimed at providing F/P/T governments and other interested stakeholders with centralized, credible information in support of evidence based policy.
Corporate Services
The Corporate Services Branch provides advice and services in relation to human resources management; facilities; procurement; health, safety and security; information technology; and information management. It is also responsible for financial planning and reporting, accounting operations, audit and evaluation, and liaising with federal central agencies on these topics.
Board Secretariat, Communications and Strategic Planning
The Board Secretariat, Communications and Strategic Planning Branch develops and manages the PMPRB’s communications, media relations, and public enquiries; manages the Board’s meeting and hearing processes, including the official record of proceedings; and coordinates activities pursuant to the Access to Information Act and the Privacy Act. It is also responsible for strategic planning and reporting.
General Counsel
The General Counsel advises the PMPRB on legal matters and leads the legal team representing Board Staff in proceedings before the Board.