The New Drug Landscape: International Availability and Pricing
PDF - 375 kb
Important new drugs have been launched since 2009, generating nearly one quarter of pharmaceutical brand-name sales in Canada by 2016. These included many new oncology medications and an increasing number of high-cost orphan drugs. In general, fewer drugs were approved for market in Canada than in the US and Europe, although Canada still ranked well internationally in terms of sales.
PMPRB’s annual Meds Entry Watch report explores the dynamics of new drugs launched in Canada and other international markets. The most recent edition focuses on new active substances (NASs) that received market approval through the US Food and Drug Administration (FDA), the European Medicines Agency (EMA) and/or Health Canada (HC) in 2015 and 2016, and analyzes their uptake, pricing, sales and availability as of the last quarter of 2016 (Q4-2016).
International markets examined include the seven countries the PMPRB considers in reviewing the prices of patented drugs (PMPRB7): France, Germany, Italy, Sweden, Switzerland, the United Kingdom (UK) and the United States (US); as well as other countries in the Organisation for Economic Co-operation and Development (OECD).
1. High-cost orphan drugs increasingly dominate the new drug landscape
In 2015, 41 new drugs received market approval in Canada and the PMPRB7, exceeding the annual average of 35 new drug launches from 2009 to 2014; 2016 was a much less active year, with 30 new active substances approved. An increasing share of new drugs received an orphan designation from the FDA and/or the EMA, reaching 54% in 2015 and 43% in 2016, a much higher share than the average of 33% in previous years. Oncology treatments continued to represent a large proportion of the new drugs: 27% of all new drugs introduced in 2015 and 2016.
New drugs launched in Canada and the PMPRB7, 2009 to 2016
Click on image for larger view

Figure description
This column graph and accompanying table illustrate the number of new drugs launched in Canada and its seven comparator countries from 2009 to 2016, as well as the share of orphan and oncology drugs.
From 2009 to 2014, an average of 35 molecules was launched each year: 33% were orphan drugs and 20% were oncology drugs.
In 2015, 41 new molecules were approved by United States Food and Drug Administration, the European Medicines Agency and/or Health Canada: 54% were orphan drugs and 34% were oncology drugs.
In 2016, 30 new molecules were approved by United States Food and Drug Administration, the European Medicines Agency and/or Health Canada: 43% were orphan drugs and 17% were oncology drugs.
2. Drugs launched since 2009 accounted for approximately one quarter of all brand-name pharmaceutical sales by Q4-2016
New drugs have a steep year-over-year uptake in sales. By Q4-2016, drugs launched in the Canadian and PMPRB7 markets since 2009 accounted for 23.8% of the total brand-name pharmaceutical market in Canada. The impact on pharmaceutical sales varied depending on the number and therapeutic relevance of the drugs launched in each particular year. For example, those launched in 2014, which included the new drugs for hepatitis C, had a greater effect on sales than the drugs launched in previous years. The sales for drugs launched in 2015 were highly concentrated, with antineoplastic drugs and antivirals together accounting for over 60% of new drug sales in Canada and the PMPRB7 in Q4-2016.
Cumulative new active substance share of brand-name drug sales, 2009–2015, Canada
Click on image for larger view

*Year of first launch in Canada and/or the PMPRB7.
Figure description
This area graph depicts the cumulative share of total brand-name drug sales in Canada for new active substances launched from 2009 to 2015 and gives the percentage of their yearly sales up to the fourth quarter of 2016. The share of sales is for Canada and PMPRB comparator countries.
blank
| Year of introduction |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
Fourth quarter of 2016 |
| 2009 |
0.05% |
0.4% |
1.1% |
2.1% |
3.0% |
3.8% |
4.4% |
5.1% |
5.3% |
| 2010 |
|
0.0% |
0.1% |
0.5% |
0.9% |
1.3% |
1.5% |
1.7% |
1.8% |
| 2011 |
|
|
0.03% |
0.7% |
1.6% |
2.2% |
3.0% |
4.2% |
4.5% |
| 2012 |
|
|
|
0.01% |
0.2% |
0.9% |
1.6% |
2.5% |
2.8% |
| 2013 |
|
|
|
|
0.02% |
1.5% |
2.5% |
3.9% |
4.1% |
| 2014 |
|
|
|
|
|
0.1% |
4.2% |
4.0% |
3.9% |
| 2015 |
|
|
|
|
|
|
0.1% |
0.6% |
1.1% |
| Total |
0.1% |
0.4% |
1.3% |
3.4% |
5.8% |
9.8% |
17.3% |
22.0% |
23.6% |
3. Fewer drugs are launched in Canada then in its PMPRB comparator countries
Fewer new drugs received market approval in 2015 through Health Canada (20) than through the FDA in the US (40) and the EMA in Europe (31), the two largest markets.
Longer term trends also show that Canada had fewer launches than most of its PMPRB comparator countries, many of which had lower average patented drug prices. Half of the new drugs launched from 2009 to 2015 had available sales in Canada by Q4-2016, placing Canada eleventh among the OECD countries. Despite this, Canada ranked third in terms of the corresponding OECD sales for these drugs (93%), suggesting that the higher-selling drugs were launched.
While this comparison reflects Canada’s relative international position in terms of drugs with available sales, it does not completely characterize the availability of new drugs. Like other countries, Canada approved additional drugs over this time period that had not yet recorded sales in MIDAS by Q4-2016; if these drugs were included, Canada’s share of new drugs launched would have increased to 62%.
Share of new drugs launched* from 2009 to 2015 and their respective share of OECD sales, Q4-2016
Click on image for larger view

*Includes all new drugs launched in Canada and the PMPRB7 during this time period.
Figure description
This is a split bar graph. For each country in the Organisation for Economic Co-operation and Development, one side of the graph gives the share of the new drugs launched in Canada and its seven PMPRB comparator countries from 2009 to 2015. The other side gives the respective share of the Organisation for Economic Co-operation and Development sales in the fourth quarter of 2016.
blank
| Country |
Share of drugs launched |
Share of sales |
| United States |
88% |
99.6% |
| Germany |
70% |
91% |
| United Kingdom |
68% |
95% |
| Austria |
63% |
92% |
| Sweden |
61% |
92% |
| Italy |
59% |
88% |
| Norway |
56% |
87% |
| Switzerland |
53% |
91% |
| Finland |
52% |
84% |
| France |
51% |
78% |
| Canada |
50% |
93% |
| Slovakia |
48% |
71% |
| Belgium |
48% |
86% |
| Slovenia |
47% |
76% |
| Japan |
43% |
80% |
| Ireland |
43% |
82% |
| Hungary |
42% |
68% |
| Australia |
40% |
84% |
| Mexico |
40% |
79% |
| Czech Republic |
39% |
50% |
| South Korea |
39% |
78% |
| Poland |
38% |
48% |
| Netherlands |
36% |
65% |
| Chile |
28% |
60% |
| Turkey |
25% |
65% |
| Luxembourg |
23% |
47% |
| Estonia |
21% |
31% |
| Greece |
21% |
33% |
| Spain |
21% |
35% |
| New Zealand |
16% |
34% |
| Portugal |
16% |
31% |
| Median value |
43% |
79% |
4. Many 2015 drugs had high treatment costs, but few demonstrated more than a moderate level of therapeutic improvement
Of the 41 new drugs approved in Canada and the PMPRB7 in 2015, more than half were designated as orphan drugs, most of which came with very high treatment costs. Many of these orphan drugs were used for the treatment of cancer. In total, oncology drugs made up approximately one-third of all of these new drugs in 2015 at an average cost of $12,000 per 28-day treatment.
Twenty of the 41 new drugs launched in 2015 received market approval in Canada, accounting for 80% of the total new drug sales by Q4-2016. In keeping with the trend in previous years, only a few of the new drugs were assessed by the PMPRB as breakthroughs or as offering a significant level of therapeutic improvement.
Click on image for larger view
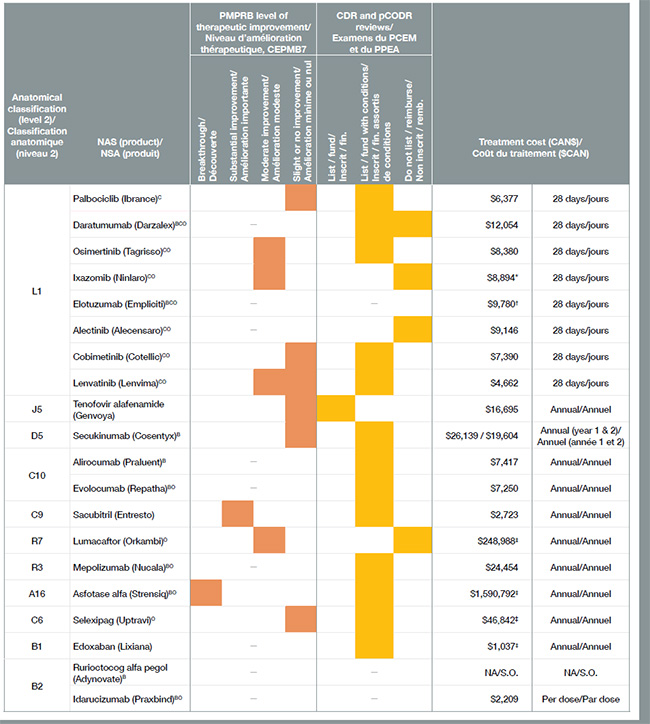
Note: Treatment costs based on Canadian list prices unless indicated: * pCODR; † median international price; ‡ CADTH.
CDR, Common Drug Review; pCODR, pan-Canadian Oncology Drug Review.
B, biologic; C, cancer; O, orphan.
Figure description
This table gives information on the 20 new drugs that received market approval in Canada in 2015.
blank
| Anatomical classification (level 2) |
New active substance (product) |
PMPRB level of therapeutic improvement |
Common Drug Review or pan-Canadian Oncology Drug Review |
Treatment cost (CAN$) |
Break-
through |
Sub-
stantial
improve-
ment |
Moderate improvement |
Slight
or no
improve-
ment |
List or Fund |
List or fund
with
conditions |
Do not list or reimburse |
| L1 |
Palbociclib (Ibrance)C |
|
|
|
X |
|
X |
|
$6,377 |
28 days |
| L1 |
Daratumumab (Darzalex)BCO |
― |
|
X |
X |
$12,054 |
28 days |
| L1 |
Osimertinib (Tagrisso)CO |
|
|
X |
|
|
X |
|
$8,380 |
28 days |
| L1 |
Ixazomib (Ninlaro)CO |
|
|
X |
|
|
X |
X |
$8,894* |
28 days |
| L1 |
Elotuzumab (Empliciti)BCO |
― |
― |
$9,780† |
28 days |
| L1 |
Alectinib (Alecensaro)CO |
― |
|
|
X |
$9,146 |
28 days |
| L1 |
Cobimetinib (Cotellic)CO |
|
|
|
X |
|
X |
|
$7,390 |
28 days |
| L1 |
Lenvatinib (Lenvima)CO |
|
|
X |
X |
|
X |
|
$4,662 |
28 days |
| J5 |
Tenofovir alafenamide (Genvoya) |
|
|
|
X |
X |
|
|
$16,695 |
Annual |
| D5 |
Secukinumab (Cosentyx)B |
|
|
|
X |
|
X |
|
$26,139/$19,604 |
Annual (year 1 and 2) |
| C10 |
Alirocumab (Praluent)B |
― |
|
X |
|
$7,417 |
Annual |
| C10 |
Evolocumab (Repatha)BO |
― |
|
X |
|
$7,250 |
Annual |
| C9 |
Sacubitril (Entresto) |
|
X |
|
|
|
X |
|
$2,723 |
Annual |
| R7 |
Lumacaftor (Orkambi)O |
|
|
X |
|
|
|
X |
$248,988‡ |
Annual |
| R3 |
Mepolizumab (Nucala)BO |
―
|
|
X |
|
$24,454 |
Annual |
| A16 |
Asfotase alfa (Strensiq)BO |
X |
|
|
|
|
X |
|
$1,590,792‡ |
Annual |
| C6 |
Selexipag (Uptravi)O |
|
|
|
X |
|
X |
|
$46,842‡ |
Annual |
| B1 |
Edoxaban (Lixiana) |
― |
|
X |
|
$1,037‡ |
Annual |
| B2 |
Rurioctocog alfa pegol (Adynovate)B |
― |
― |
NA |
NA |
| B2 |
Idarucizumab (Praxbind)BO |
― |
― |
$2,209 |
Per dose |
Note: Treatment costs based on Canadian list prices unless indicated: * pCODR; † median international price; ‡ CADTH.
CDR, Common Drug Review; pCODR, pan-Canadian Oncology Drug Review.
B, biologic; C, cancer; O, orphan.
Note: Drug costs do not reflect rebates resulting from confidential product listing agreements.
Source: Sales data from QuintilesIMS MIDAS™ database. All rights reserved.
Disclaimer: Although based in part on data provided under license by the QuintilesIMS MIDAS™ Database, the statements, findings, conclusions, views and opinions expressed in this report are exclusively those of the PMPRB and are not attributable to QuintilesIMS.
NPDUIS is a research initiative that operates independently of the regulatory activities of the PMPRB.