Annual Report 2006

Table Of Contents
- Mission and Values
- Letter to The Minister of Health
- Highlights 2006
- Chairperson’s Message
- About The Patented Medicine Prices Review Board: Mandate and Jurisdiction
- Governance
- Regulating Prices of Patented Medicines
- Compliance and Excessive Price Guidelines
- Voluntary Compliance Undertakings
- Hearings
- The Federal Court of Canada Decision in the Matter of LEO Pharma Inc. and the Patented Medicine Dovobet
- Reporting Information on Key Pharmaceutical Trends
- Trends in Sales of Patented Drugs
- Price Trends
- Comparison of Foreign Prices to Canadian Prices
- Utilization of Patented Drugs
- Manufacturing Trends in Canada
- Canadian Sales in the Global Context
- Analysis of Research-and-Development Expenditure
- National Prescription Drug Utilization Information System
- Monitoring and Reporting of Non-Patented Prescription Drug Prices
- Amendments to the Patented Medicines Regulations, 1994
- Review of the Board's Excessive Price Guidelines
- Communications
- Glossary
- Annex 1 - Criteria for Commencing an Investigation
- Annex 2 - Patented Drug Products Introduced in 2006 (1)
- Annex 2 - Patented Drug Products Introduced in 2006 (II)
- Annex 3 - Research & Development
The mission of the Patented Medicine Prices Review Board (PMPRB) is to contribute to Canadian health care by ensuring that prices of patented medicines are not excessive and by analyzing and reporting to Canadians on price trends of all medicines and on research and development conducted by patentees. The PMPRB achieves this by:
- promoting voluntary compliance with the Guidelines established by the Board;
- reviewing prices and taking remedial action when necessary;
- consulting with interested parties on Guidelines and other matters of policy; and
- fostering awareness of the Board´s mandate, activities and achievements through communication, dissemination of information and public education.
In fulfilling the mission we are committed to innovative leadership based on the following values:
- effectiveness and efficiency;
- fairness;
- integrity;
- mutual respect;
- transparency;
- a supportive and challenging work environment.
To obtain our publications, log on to our Web site at www.pmprb-cepmb.gc.ca, or call us at our toll-free number: 1 877 861-2350
The Patented Medicine Prices Review Board
Standard Life Centre
Box L40
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Facsimile: (613) 952-7620
TTY: (613) 957-4373
All PMPRB publications are available in both official languages.
Catalogue number: H78-2005; ISBN: 0-662-69593-3
PDF: Catalogue number: H78-2005E-PDF; ISBN: 0-662-42565-0

REGULATORY MANDATE
The PMPRB's regulatory activities continued to increase in 2006.
Compliance
- Ninety nine new patented drug products (DINs) for human use were reported to the PMPRB in 2006 of which 29 medicines, representing 43 DINs, were new active substances. As of March 31, 2007, 79 new patented drug products had been reviewed. Of those, 68 were considered to be within the Guidelines, while 11 are subject to ongoing investigations.
- A total of 1181 patented drug products for human use were under the PMPRB's jurisdiction in 2006.
Enforcement
- The Board approved four Voluntary Compliance Undertakings, including one in May 2007 in the context of a hearing.
- The Board issued eight Notices of Hearing, bringing the total of ongoing proceedings to ten. The Dovobet and Nicoderm matters, initiated in previous years, remain before the hearing panels for final resolution.
REPORTING MANDATE
The PMPRB continues to enhance the pharmaceutical trends section of the Annual Report, with, again this year, more in-depth analysis of the key pharmaceutical indices. In addition, the PMPRB has published a number of studies and reports – the Guidelines for Conducting Pharmaceutical Budget Impact Analysis, under the National Prescription Drug Utilization Information System; and the Canadian and Foreign Price Trends and the Trends in Canadian Sales and Market Structure reports under the Non-Patented Prescription Drug Prices initiative.
Sales Trends
- Sales of patented drugs in Canada increased by 3.7% to $12 billion in 2006. Annual growth in sales of patented drugs stood at 27.0% in 1999 and remained in double digits until 2003.
- The share of total sales accounted for by patented drugs declined to 68.1% in 2006 from 71.4% in 2005.
- The antineoplastics and immunomodulating agents (such as drugs used in chemotherapy) remain the leading contributing drug class to sales growth.
Price Trends
- Prices in Canada – the manufacturers' prices of patented drugs, as measured by the Patented Medicine Price Index (PMPI), decreased on average by 0.2% in 2006. The slight decline in the PMPI is attributable to falling prices paid by hospitals. Again this year, the PMPI varied by class of customer (hospitals, pharmacies, wholesalers) and across the provinces and territories.
- The Consumer Price Index (CPI) was at 2.0% over the same period. Analysis of prices by therapeutic class demonstrates considerable variability in price changes.
- Foreign-to-Canadian prices – the ratio of Canadian prices to the international median for comparator countries was slightly below parity. Five of the seven comparator countries registered overall price declines in 2006 while US prices rose by more than 7% on average.
Research and Development
- Patentees reported total R&D expenditures of $1.210 billion in 2006, a slight decrease of 1.9% over the previous year. Rx&D members reported R&D expenditures of $949 million in 2006 compared to $1.0 billion in 2005.
- The R&D-to-sales ratio continues its downward trend with 8.1% from 8.7% in 2005, as did the R&D-to-sales ratio for members of Rx&D – 8.5% compared to 8.8% in the previous year. The ratios have been below 10% since 2001 and 2003 respectively.
- Patentees reported spending $232.4 million on basic research, representing 20.0% of current R&D expenditures. Basic research increased by 8.0% in 2006 relative to 2005.
Consultations
The Board initiated stakeholder consultations on its Excessive Price Guidelines. The review of the Guidelines is ongoing with the upcoming creation of working groups and bilateral meetings in the fall with representatives of governments, consumers and the industry, as reported in a Board communiqué issued on May 31, 2007 and posted on the PMPRB Web site.
The past year has been one of change and challenge for the PMPRB, as well as for myself as I accepted the honour of being appointed to the position of Chairperson of the Board.
During 2006, the work of the PMPRB has been shaped by a variety of factors, including an increase in the number of Notices of Hearing issued under the Patent Act, the launch of a comprehensive review of, and public consultation on, our Excessive Price Guidelines, and several new studies and analyses relating to the Board's responsibilities under the National Prescription Drug Utilization Information System (NPDUIS) and the monitoring and reporting on Non-Patented Prescription Drug Prices (NPPDP). As this report shows, all of these new activities have been carried out in addition to our core activities, which include the review of the prices of more than 1100 patented drug products, of which 99 are new drugs that came under our jurisdiction in 2006.
The Board issued six Notices of Hearing in 2006 to determine whether certain patented medicines are, or were being, sold in any market in Canada at prices that, in the opinion of the Board, are or were excessive. Two more Notices were issued over the last few months. This number is equal to the total of eight Notices of Hearing issued by the Board from its inception in 1987 through to 2005.
As reported in the 2005 Annual Report, the Board followed through on its plans to issue a discussion guide, and to hold face-to-face consultations with stakeholders on its Excessive Price Guidelines. The fundamental goal of this review is one that is critical to the work of the PMPRB. The Board wants to ensure that the Guidelines remain relevant and provide appropriate guidance to Board Staff, patentees and other interested stakeholders, on how the price review process is conducted. Furthermore, it is important that the Guidelines reflect the Board's interpretation, in the present day context and for the future, of the price determination factors set out in the Patent Act.
The key issues being addressed by this review of the Guidelines include the categorization of new drugs, price tests, the definition of "any market," "re-benching" (i.e., relating to whether re-setting the benchmark price may be appropriate), and, guiding principles for the price review process as these relate to the mandate of the Board.
The Board is continuing its analysis. To further advance this work, the Board will hold bilateral consultations with groups representing sectors of the pharmaceutical industry, federal/ provincial/territorial governments and consumers, in the fall of 2007.
Another important area of activity for the Board relates to the analytical and technical support we provide to Federal/Provincial/Territorial Ministers of Health under the NPDUIS. Recently, we released the Budget Impact Analysis Guidelines and the New Drug Pipeline Monitor. In the context of our monitoring of non-patented prescription drug prices, we released two reports in 2006, Canadian and Foreign Price Trends, and Trends in Canadian Sales and Market Structure. Shortly, we will release the report on Market for New Off-Patented Drugs. Through the publishing of these quarterly reports, the Board is helping to fill an information gap, and thus assist policy makers to better understand trends in non-prescription drug prices, and the factors influencing drug costs in Canada.
The PMPRB is increasingly being challenged to respond to new demands, through the review of the Excessive Price Guidelines, acting in the public interest by holding public hearings into specific matters of potential excessive pricing, and a host of other activities. However, the commitment, dedication and expertise of Board Members and Board Staff, help ensure our ability to effectively meet these challenges, to serve Canadians, and to contribute to the integrity of the health care system.

Brien G. Benoit, MD
Chairperson
The Patented Medicine Prices Review Board is an independent quasi-judicial body established by Parliament in 1987 under the Patent Act (Act). The Minister of Health is responsible for the pharmaceutical provisions of the Act as set out in sections 79 to 103.
Although part of the Health Portfolio, the PMPRB carries out its mandate at arms-length from the Minister of Health1. It also operates independently of other bodies such as Health Canada, which approves drugs for safety and efficacy, and public drug plans, which have responsibility for approving the listing of drugs on their respective formularies for reimbursement purposes.
MANDATE
The PMPRB has a dual role:
Regulatory
To ensure that prices charged by patentees for patented medicines sold in Canada are not excessive thereby protecting consumers and contributing to Canadian health care.
Reporting
To report on pharmaceutical trends of all medicines, and on R&D spending by pharmaceutical patentees thereby contributing to informed decisions and policy making.
JURISDICTION
Regulatory
The PMPRB is responsible for regulating the prices that patentees charge – the factory-gate price – for prescription and non-prescription patented drugs sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, to ensure that they are not excessive. The PMPRB regulates the price of each patented drug product, including each strength of each dosage form of each patented medicine sold in Canada. This is normally the level at which Health Canada assigns a Drug Identification Number (DIN).
Health Canada assesses new medicines to ensure that they conform to the Food and Drugs Act and the Food and Drug Regulations. Formal authorization to market or distribute a medicine is granted through a Notice of Compliance (NOC). A medicine may be temporarily distributed with specified restrictions before receiving a NOC, as an Investigational New Drug or under Health Canada's Special Access Program.
The PMPRB has no authority to regulate the prices of non-patented drugs, and does not have jurisdiction over prices charged by wholesalers or retailers, or over pharmacists' professional fees. Also, matters such as whether medicines are reimbursed by public drug plans, distribution and prescribing are outside the purview of the PMPRB.
Under the Patented Medicines Regulations, 1994, patentees are required to file price and sales information twice a year for each strength of each dosage form of each patented medicine sold in Canada for price regulation purposes. Patentees are also required to file R&D expenditures once a year for reporting purposes.
Patentees are also required to inform the PMPRB of their intention to sell a new patented medicine. They are not required to obtain approval of the price of a patented medicine before it is sold, but they are required to comply with the Act to ensure that prices of patented medicines sold in Canada are not excessive. In the event that the Board finds, after a public hearing, that a price is or was excessive in any market it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Reporting
The PMPRB reports annually to Parliament, through the Minister of Health, on its activities, on pharmaceutical trends relating to all medicines, and on the R&D spending by pharmaceutical patentees. In addition to these reporting responsibilities, under Section 90 of the Act, the Minister of Health has the authority to direct the PMPRB to inquire into any other matter. Under this provision, the Minister has directed the Board to undertake two initiatives: the National Prescription Drug Utilization Information System, and monitoring and reporting on Non-Patented Prescription Drug Prices.
National Prescription Drug Utilization Information System (NPDUIS)
In 2001, pursuant to an agreement by the Federal/Provincial/Territorial Ministers of Health, the federal Minister of Health directed the PMPRB to conduct research under the National Prescription Drug Utilization Information System (NPDUIS). The purpose of the NPDUIS is to provide critical analyses of price, utilization and cost trends so that Canada's health system has more comprehensive and accurate information on how prescription drugs are being used and on sources of cost increases.
Non-Patented Prescription Drug Prices (NPPDP)
In 2005, the Minister of Health, on behalf of himself and his provincial and territorial colleagues, directed the PMPRB to monitor and report on the prices of non-patented prescription drugs. This function is aimed at providing a centralized credible source of information on non-patented drug prices in support of the priority focus of the National Pharmaceuticals Strategy regarding pricing and purchasing.
1 The Health Portfolio contributes to specific dimensions of improving the health of Canadians. It comprises Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission, the Assisted Human Reproduction Agency of Canada and the Patented Medicine Prices Review Board.
The Board consists of not more than five members who serve on a parttime basis, appointed by the Governor-in-Council, including a Chairperson and a Vice-Chairperson. The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB with the authority and responsibility to supervise and direct its work. The Executive Director manages the work of the Staff. In addition to the Executive Director, Senior Staff consists of the Director of Compliance and Enforcement, the Director of Policy and Economic Analysis, the Director of Corporate Services, the Secretary of the Board, and Senior Counsel.
Since the beginning of 2006, the Minister of Health announced three appointments to the Board: Dr. Brien G. Benoit as Chairperson, Ms. Mary Catherine Lindberg as Vice- Chairperson, and Ms. Anne Warner La Forest as Member, bringing the Board to its full complement.
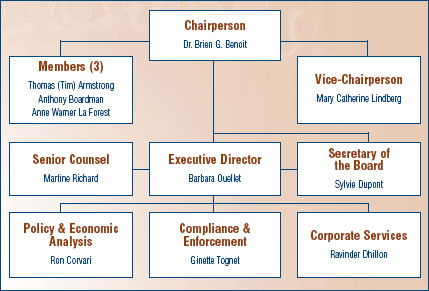
MEMBERS OF THE BOARD

Chairperson
Brien G. Benoit, BA, MD, MSc, FRCSC, FACS
Dr. Brien G. Benoit was first appointed Member of the PMPRB in May 2005. In October of the same year, Dr. Benoit became Vice-Chairperson and took on the responsibilities as Chairperson until his appointment as Chairperson in June 2006.
A Neurosurgeon, Dr. Benoit is on the Active Attending Staff of the Ottawa Hospital. Dr. Benoit is also a Professor of Neurosurgery at the University of Ottawa. Throughout his career, Dr. Benoit has held several administrative positions including Chief of Staff of the Ottawa Civic Hospital, from 1996 to 1998; Program Director, Neurosurgery at the University of Ottawa, from 1995 to 2003; Chair of Neurosurgery, at the University of Ottawa, from 1997-2003; and Deputy Surgeon-in-Chief at the Ottawa Hospital (Civic Campus) from 2002 to 2004. Dr. Benoit was also Chair of the Operating Room Committee at the Ottawa Hospital (Civic Campus), from 1993 to 2004. Dr. Benoit has published extensively in leading academic journals. He has received several awards, including Best Surgical Teacher from the Department of Surgery of the University of Ottawa in 1991 and 2000.
In addition to being a Fellow of the Royal College of Surgeons (Neurosurgery), Dr. Benoit is a member of several professional associations including the Canadian Medical Association, the Ontario Medical Association, the Royal College of Physicians and Surgeons of Canada, and the American College of Surgeons, to name a few.
Vice-Chairperson
Mary Catherine Lindberg, BSP
Mary Catherine Lindberg was appointed Member and Vice- Chairperson of the Board in June 2006.
Ms. Lindberg is currently the Executive Director of the Ontario Council of Academic Hospitals, an organization of 25 Academic Hospitals that are fully affiliated with a University and their Faculty of Medicine. Prior to retiring from the Ministry of Health and Long Term Care, she was an Assistant Deputy Minister with responsibilities for registration and eligibility for the Ontario Health Insurance Plan (OHIP), payment to physicians, the Ontario Drug Program and the Laboratories.
Some of her major activities were the development and introduction of the Trillium Drug Program, leading negotiations for the government for physicians, pharmacists, chiropractors, physiotherapists, optometrists and private laboratory owners.
Ms. Lindberg has a degree in pharmacy from the University of Saskatchewan and has her pharmacist's licence in both the provinces of Saskatchewan and Ontario.
Members
Thomas (Tim) Armstrong, BA, LLB, QC, O. Ont.
Tim Armstrong was appointed Member of the Board in October 2002.
Mr. Armstrong practiced law from 1958 to 1974, first in the Civil Litigation Division of the federal Department of Justice, subsequently in private practice in Toronto with Jolliffe, Lewis & Osler and later as senior partner of Armstrong & MacLean, specializing in administrative law litigation, presenting cases to administrative tribunals, the Ontario courts, the Federal Court, and the Supreme Court of Canada.
In 1974, he began his career as a senior Ontario public servant as Chair of the Ontario Labour Relations Board (1974-1976), Deputy Minister of Labour (1976-1986), Agent General for Ontario in Tokyo (1986-1990), and Deputy Minister of Industry, Trade and Technology (1991-1992). He was advisor to the Premier of Ontario on Economic Development from 1992 to 1995. Mr. Armstrong was counsel to the law firm McCarthy Tétrault from 1995 to 2002. In the 1990s, he served as a member on the boards of directors of Algoma Steel, deHavilland Aircraft and Interlink Freight.
He has been Chief Representative for Canada for the Japan Bank for International Cooperation since 1996 and also serves as arbitrator and mediator by consensual, provincial and federal government appointment in the field of labour relations. In his dispute resolution work, he was appointed facilitator/mediator by the Ontario Health Services Restructuring Commission from 1998-1999. Subsequently, in 2002-2003, he was designated by the Ontario government as mediator/arbitrator under the City of Toronto Labour Disputes Resolution Act, 2002.
He is currently the Chair of the Radiation Safety Institute of Canada and Vice-Chair of the Ontario Press Council.
Mr. Armstrong was awarded the Order of Ontario in 1995 in recognition of his contribution to public service in Ontario.
Anthony Boardman, BA, PhD
Dr. Boardman was appointed Member of the Board in January 1999 and was re-appointed in March 2005.
Dr. Boardman is the Van Dusen Professor of Business Administration in the Strategy and Business Economics Division of the Sauder School of Business at the University of British Columbia (UBC). He graduated from the University of Kent at Canterbury (BA, 1970), and Carnegie- Mellon University (PhD, 1975). Prior to taking up his position at UBC he was a professor at the Wharton School, University of Pennsylvania.
His current research interests include public-private partnerships, cost-benefit analysis and strategic management. Dr. Boardman has been a consultant to many private and public organizations including Vodafone, Stora Enzo, PricewaterhouseCoopers, the Treasury of New Zealand and all levels of government in Canada. He has taught executive programs in Finland, China, Australia and elsewhere, and has won a number of teaching awards. As a member of the MBA Core Team at UBC, he won the Alan Blizzard award. Between 1995 and 2001, Dr. Boardman was a member of the Pharmacoeconomic Initiative Scientific Committee in BC. Currently, he is a member of the National Academies Committee on Medical Isotope Production Without Highly Enriched Uranium.
During his career, Dr. Boardman has published many articles in leading academic journals. Recently, he completed the third edition of Cost- Benefit Analysis: Concepts and Practice.
Anne Warner La Forest, LLB (UNB), LLM (Cantab)
Anne Warner La Forest was appointed Member of the Board in March 2007.
Ms. La Forest is currently a law professor at the University of New Brunswick. Member of the New Brunswick Securities Commission since 2004, she is also the Chair of the Commission's Human Resources Committee.
After working in private practice with the firm of Fraser & Beatty in Toronto for several years, Anne La Forest joined the Faculty of Law at Dalhousie University in 1991. In 1996, she was appointed Dean of the New Brunswick University Faculty of Law, a position she held until 2004.
A member of the bars of New Brunswick, Nova Scotia and Ontario, Ms. La Forest has extensive experience as an arbitrator and has acted as a consultant on matters relating to human rights, employment, property and extradition law. She has been a member of the Nova Scotia Human Rights Tribunal, a member of the Social Sciences and Humanities Research Council and Chair of the Fellowships Committee. She has also served as Arbitrator in the province of Nova Scotia as well as Commissioner of the province's Human Rights Commission. She is a Fellow of the Cambridge Commonwealth Society and is currently a member of the Board of Governors of the National Judicial Institute.
She holds an honours degree in International law from Cambridge University in the United Kingdom.
Ms. La Forest has published many articles, books and case comments during her career and has been the chair or has served as a panelist at many national and international law conferences.
BUDGET
The PMPRB operated with a budget of $11,690,025 in 2006-2007 and an approved staff level of 62 employees. The budget included resources for the National Prescription Drug Utilization Information System (NPDUIS) and for the monitoring and reporting on Non-Patented Prescription Drug Prices in Canada (NPPDP) under the National Pharmaceuticals Strategy (NPS).
In June 2006, the PMPRB received additional funding for the years 2006-2007 and 2007-2008, bringing its total budget to $11.6 million from its initial allocated budget of $6.5M. These additional funds were allocated to the PMPRB to conduct public hearings to determine whether, under Sections 83 and 85 of the Patent Act, certain patented medicines are being sold, or were sold, in any market in Canada at prices that, in the Board's opinion, are, or were, excessive; and, to review the Board's Excessive Price Guidelines which serve as a tool in the establishment of non-excessive prices for patented medicines sold in Canada.

- Compliance and Excessive Price Guidelines
- Voluntary Compliance Undertakings
- Hearings
- The Federal Court of Canada Decision in The Matter of Leo Pharma Inc. and The Patented Medicine Dovobet
Under section 82 of the Patent Act(Act), pharmaceutical patentees are required to notify the PMPRB of their intention to offer a patented drug product for sale and the date on which they expect to begin selling it. Under the Patented Medicines Regulations, 1994 (Regulations), patentees are subsequently required to:
- file a Medicine Identification Sheet (Form 1) within 30 days after either the issuance of a Notice of Compliance or the date on which the patented drug product was first sold in Canada, whichever comes first;
- report information on the introductory prices and sales of new patented drug products (Form 2), within 60 days of the date of first sale; and
- continue to file detailed information on prices and sales of each patented drug product for the first and last six-month period of each year (Form 2), 30 days after the end of each period, i.e., on July 30 and January 30 respectively, for as long as the drug product remains under the Board's jurisdiction.
The PMPRB reviews the pricing information for all patented medicines sold in Canada on an ongoing basis to ensure that the prices charged by patentees comply with the Excessive Price Guidelines (Guidelines) established by the Board. The Guidelines are published in the PMPRB's Compendium of Guidelines, Policies and Procedures.2
Excessive Price Guidelines
The Guidelines are based on the price determination factors in section 85 of the Act and have been developed by the Board in consultation with stakeholders, including the provincial and territorial Ministers of Health, consumer groups and the pharmaceutical industry. In summary, the Guidelines provide that:
- prices for most new patented drug products are limited such that the cost of therapy for the new drug does not exceed the highest cost of therapy for existing drugs used to treat the same disease in Canada;
- prices of new breakthrough patented drug products and those that bring a substantial improvement are generally limited to the median of the prices charged for the same drug in other industrialized countries listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States);
- price increases for existing patented drug products are limited to changes determined by the Board's Consumer Price Index (CPI) methodology; and
- price of a patented drug product in Canada may, at no time, exceed the highest price for the same drug in the foreign countries listed in the Regulations.
Board Staff reviews the prices of all patented drug products sold in Canada. When it finds that the price of a patented drug product appears to exceed the Guidelines, and the circumstances meet the criteria for commencing an investigation, Board Staff will conduct an investigation to determine if the price of the patented drug product in fact exceeds the Guidelines. Additional information on the criteria for commencing an investigation is available in Annex 1 on page 54. An investigation could result in:
- its closure where it is concluded that the price was within the Guidelines;
- a Voluntary Compliance Undertaking (VCU) by the manufacturer to reduce the price and take other measures to comply with the Guidelines including the repayment of excess revenues obtained as a result of excessive prices; or
- a public hearing to determine if the price is excessive and to make any remedial order determined by the Board.
As part of the PMPRB's transparency initiative, the list of New Patented Medicines Reported to the PMPRB is posted on our Web site every month. This list includes information on the status of the review (i.e., under review, within Guidelines, VCU, Notice of Hearing). As reported in the April 2005 NEWSletter, beginning in 2005, drugs that are the subject of an investigation are reported as such – they are no longer reported as "under review". When the price appears to exceed the Guidelines and where the criteria for commencing an investigation have been triggered, these drug products are identified as "under investigation".
TOP
Failure to Report
In order to fulfill its regulatory mandate, as described on page 2, the PMPRB relies upon the patentees' full and timely disclosure of any and all medicines being sold in Canada to which a patent pertains.
Late filing by patentees is an important issue because it may delay the price review. Although, in most cases, patentees ultimately comply with the filing requirements, an issue exists regarding a number of patentees' failure to report complete information within the time frames specified in the Regulations. In 2006, twelve new drug products (or DINs) were first reported to the PMPRB although they were patented and sold prior to 2006.
Ifex, Procytox, Uromitexan, PhosLo, FSME-Immun, Varivax III, Apo-Salvent CFC Free and Ratio-Salbutamol HFA were patented and sold in Canada prior to being reported as being under the PMPRB's jurisdiction. They are currently being sold by Baxter Corporation, Prempharm Inc., Merck Frosst Canada Ltd., Apotex Inc. and Ratiopharm.
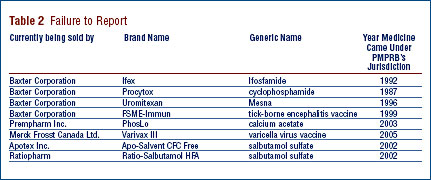
Failure to File (FTF)
The Board is pleased to report that there were no Board Orders issued for the January to June 2006 and the July to December 2006 filing periods.
It is a patentee's statutory responsibility to ensure complete information is filed within the statutory time frame.
Information on the statutory reporting requirements is available in the Act, the Regulations, the Compliance and Enforcement Policy of the Guidelines, and the Patentees' Guide to Reporting, all of which can be found on our Web site under Legislation, Regulations and Guidelines.
Human Drug Advisory Panel (HDAP)
The Board established the HDAP to provide recommendations for the categorization of new drug products and the selection of comparable drug products.
The mandate of the HDAP is to provide credible, independent and expert scientific advice to the PMPRB respecting the development and application of the Guidelines related to the scientific evaluation of patented medicines. The approach is evidence-based and the recommendations reflect medical and scientific knowledge and current clinical practice.
The HDAP is comprised of 3 members
- Dr. Jean Gray, MD, FRCPC, Professor Emeritus of medical education, medicine and pharmacology at Dalhousie University;
- Dr. Mitchell Levine, MD, MSc, FRCPC, FISPE, Professor, Department of Clinical Epidemiology and Biostatistics, St. Joseph's Healthcare Hamilton Centre for Evaluation of Medicines; and
- James McCormack, BSc(Pharm), Pharm D, Professor of Pharmaceutical Sciences, University of British Columbia.
During 2006, the HDAP reviewed a total of 34 drug products.
New Patented Drug Products in 2006
There were 99 new patented drug products, or DINs, for human use introduced in 2006. Some are one or more strengths of a new active substance (NAS) and others are new presentations of existing medicines.
For purposes of our price review, a new patented drug product in 2006 is defined as any patented drug product introduced in Canada, or previously marketed but first patented between December 1, 2005 and November 30, 2006.3
Figure 1 below provides information on new patented drug products for human use from 1988 to 2006.
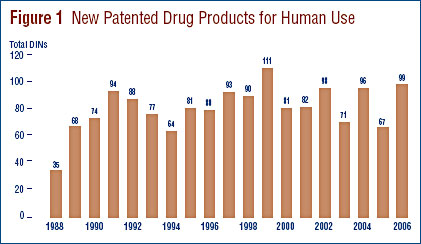
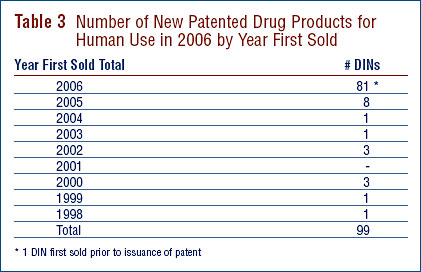
Nineteen (19%) of the 99 new patented DINs were being sold in Canada prior to the issuance of a Canadian patent which brought them under the PMPRB's jurisdiction. These DINs are denoted by a "FPG" (first patent granted) in Annex 2 on page 55. Table 3 identifies the number of patented drug products by the year in which they were first sold. The time delay between date of first sale and date of patent grant for these products ranged from several months to eight years.
New Active Substances in 2006
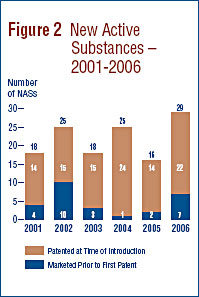 A new active substance (NAS) may include more than one DIN if it is sold in more than one strength or dosage form. In 2006, there were 29 NASs marketed as 43 DINs. As shown in Figure 2 and Table 4, seven of the 29 patented NASs that came under the PMPRB's jurisdiction were sold prior to 2006.
A new active substance (NAS) may include more than one DIN if it is sold in more than one strength or dosage form. In 2006, there were 29 NASs marketed as 43 DINs. As shown in Figure 2 and Table 4, seven of the 29 patented NASs that came under the PMPRB's jurisdiction were sold prior to 2006.
The PMPRB's list of patented NASs in any year may differ from the list of NASs approved by Health Canada's Therapeutic Products Directorate (TPD) for the following reasons:
- the NAS is not patented and therefore not subject to the PMPRB's jurisdiction;
- the NAS may not be on the TPD list because it is being sold under the Special Access Program (SAP) before it receives a Notice of Compliance (NOC); or
- the NAS may have been approved, but is not being sold.
Health Canada reported 16 NASs in 2006 but not all were introduced to the market in that year.4
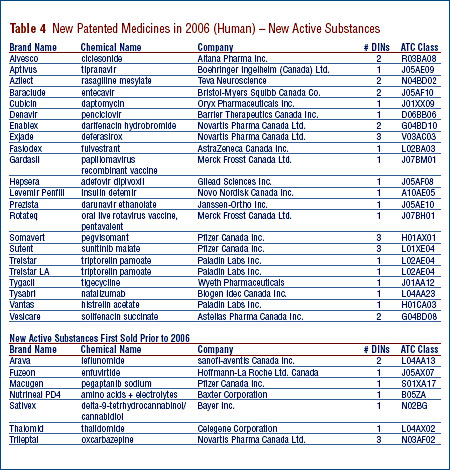
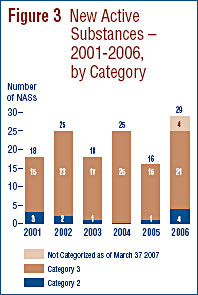 Figure 3 provides a breakdown of the patented NASs for human use, by category assigned for price review purposes, over the six-year period 2001 through 2006 inclusive.5
Figure 3 provides a breakdown of the patented NASs for human use, by category assigned for price review purposes, over the six-year period 2001 through 2006 inclusive.5
Summary Reports of the price reviews of NASs are posted on the PMPRB Web site when the price review is completed and the price is within the Guidelines
Price Review of New Patented Drugs for Human Use
A list of the 99 new patented drug products and their price review status at the time of this report appears in Annex 2 on page 55. Of the 99 new patented DINs:
- the prices of 79 had been reviewed as of March 31, 2007;
- 68 were found to be within the Guidelines;
- 11 were priced at levels which appeared to exceed the Guidelines and investigations were commenced. For a more detailed explanation of the criteria for commencing an investigation, please refer to Annex 1 on page 54; and
- 20 DINs are still under review.
Price Review of Existing Patented Drugs for Human Use
For the purpose of this report, existing medicines include all patented drug products that were first sold and reported to the PMPRB prior to December 1, 2005. The Guidelines limit the price changes for existing patented drugs to changes in the Consumer Price Index (CPI) methodology developed by the Board. In addition, the price of a patented drug cannot exceed the highest price of the same drug product in the countries listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, the United Kingdom, and the United States).
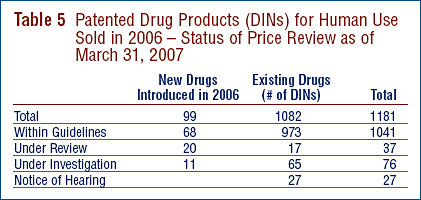
At the time of this report, there were 1082 existing patented drug products (or DINs):
- the prices of 973 existing DINs (89.9%) were within the Guidelines;
- 59 existing DINs were the subject of investigations (see paragraph above for price tests applied to existing drug products);
- 41 were opened in 2006
- 16 were opened in 2005
- two were opened in 2003
- Two were opened in 2004 as a result of introductory pricing;
- Four were opened in 2005 as a result of introductory pricing;
- 27 DINs – Nicoderm (three DINs), Dovobet, Adderall XR (six DINs), Risperdal Consta (three DINs), Airomir, Copaxone, Concerta (four DINs), Strattera (five DINs), Quadracel, Pentacel and Penlac – were, or are currently, the subject of a hearing under section 83 (see Hearings, on page 16); and,
- 17 DINs were still under review.
A summary of the review, compliance and investigation status of the new and existing patented drug products for human use in 2006 is provided in Table 5.
CDR / PMPRB
The Common Drug Review (CDR) is a single process for reviewing new drugs and providing formulary listing recommendations to participating publicly-funded federal, provincial and territorial drug benefit plans in Canada. All jurisdictions are participating in the CDR except Quebec.
The CDR reviews new drugs and provides an evidence-based formulary listing recommendation, made by the Canadian Expert Drug Advisory Committee (CEDAC). The drug plans consider the CEDAC recommendation and also their individual plan mandates, priorities and resources when making formulary listing and coverage decisions. More information on CDR and CEDAC is available from the Canadian Agency for Drugs and Technologies in Health (CADTH) Web site (http://www.cadth.ca).
Table 6 provides information on CDR reviews and on the PMPRB price reviews. The CDR reviews drug products following issuance of NOC. The PMPRB reviews all patented medicines sold in Canada. A medicine may be sold prior to the issuance of a patent. As such, it would not be under the PMPRB's jurisdiction.
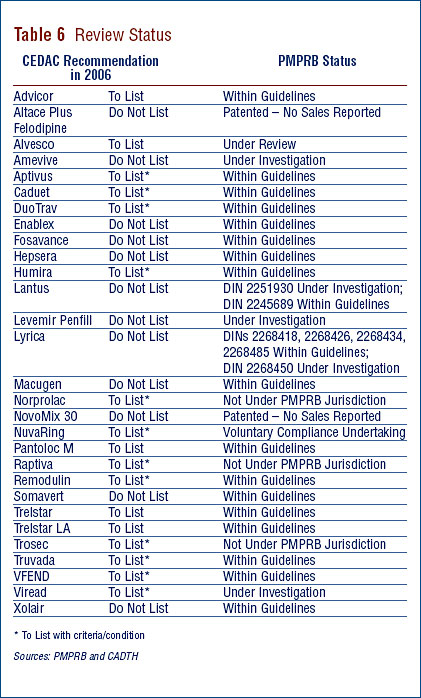
Update of New Patented Drug Products reported in previous Annual Reports
Table 7 provides an update of the review status of new patented medicines, at the DIN level, reported in previous years' Annual Reports.6
Update of Existing Medicines from the 2005 Annual Report
In last year's Annual Report, it was reported that, of the 969 existing patented drug products for human use sold in 2005, the prices of 22 were still under review. The results of those reviews concluded that: six DINs were within the Guidelines; ten DINs were priced at levels that appeared to exceed the Guidelines and therefore investigations were initiated; and six are still under review and included in the total figure of existing drugs under review reported in Table 5, on page 13. In its 2005 Report, the PMPRB had also reported that 37 DINs were under investigation6. Of those, 11 investigations have been concluded: in eight cases the prices were ultimately found to be within the Guidelines; and for three cases, VCUs were approved: Eloxatin (2 DINs), Hextend. (See Voluntary Compliance Undertakings on page 15.) Six DINs were subject to a Notice of Hearing: Penlac, Concerta (3 DINs), Quadracel and Pentacel. Twenty are still under investigation and included in the total figure of existing drugs under investigation reported in Table 5 on page 13. Also in last year's Annual Report it was reported that 15 DINs were the subject of a Notice of Hearing (NoH) and, at the time of this report, these hearings are ongoing.
Patented Drugs for Veterinary Use
The complaints-driven approach for regulating the prices of patented drugs for veterinary use remains in place. Board Staff only reviews the introductory prices of new patented veterinary medicines. Existing medicines are subject to review only when a complaint with significant evidence has been received. No complaints were received in 2006.
In last year's Annual Report it was reported that all drugs for veterinary use had been reviewed and found to be within the Guidelines. One drug product, Paylean 20, sold by Elanco Animal Health Canada, a division of Eli Lilly and Company, was sold prior to being reported to the PMPRB in 2006 and is under review. In 2006, six new DINs were reported to the PMPRB. These are under review. The summary reports of the price reviews of drug products for veterinary use are made available on the PMPRB's Web site under Regulatory; Patented Medicines; Reports on New Patented Drugs for Veterinary Use.
2 The Compendium of Guidelines, Policies and Procedures (Compendium) is available on the PMPRB's Web site under Legislation, Regulations and Guidelines, or by calling the toll-free number: 1 877 861-2350.
3 Because of the timing of the filing requirements under the Patented Medicines Regulations, 1994 and the manner of calculating benchmark prices, drug products introduced or patented in December are considered to be new patented products in the following year.
4 Annual Drug Submission Performance Report, Section 4, January-December 2006, Therapeutic Products Directorate, Health Canada.
5 For purposes of conducting introductory price reviews, the PMPRB categorizes new drug products as follows:
- Category 1 - a new DIN of an existing or comparable dosage form of an existing medicine, usually a new strength of an existing drug (line extension).
- Category 2 - the first drug to treat effectively a particular illness or which provides a substantial improvement over existing drug products, often referred to as "breakthrough" or "substantial improvement".
- Category 3 - a new drug or new dosage form of an existing medicine that provides moderate, little or no improvement over existing medicines.
For complete definitions of the categories, refer to the Compendium of Guidelines, Policies and Procedures, Chapter 3, section 3, page 23.
6 In the 2005 Annual Report, 37 DINs were subject to an investigation. The information included about these 37 drug products (DINs) should have been reported as follows – 27 investigations were opened in 2005 (3 pertaining to existing drug products); 1 investigation in 2004; 2 investigations in 2003; and 7 investigations pertaining to new drug products (4 investigations in 2004 as a result of introductory pricing; and, 3 investigations in 2003 as a result of introductory pricing).
A Voluntary Compliance Undertaking (VCU) is a written undertaking by a patentee to adjust its price to conform to the Excessive Price Guidelines. Detailed information and definitions are available in the Glossary of this Report on page 51.
Under the Compliance and Enforcement Policy, patentees are given an opportunity to submit a VCU when Board Staff concludes, following an investigation, that the price set forth by the patentee for a patented medicine sold in Canada appears to have exceeded the Board's Excessive Price Guidelines (Guidelines).
Publication of VCU
It has been the practice of the Board to publish VCUs upon their approval by the Chairperson or the Board. Once a patentee has been informed that the terms of a VCU have been approved, the document becomes public. In the context of the PMPRB's policy on compliance and enforcement, VCUs are posted on our Web site, reported in our NEWSletter, and included in the Annual Report.
Approval of a VCU by the Chairperson is an alternative compliance mechanism to the commencement of formal proceedings through the issuance of a Notice of Hearing.
Under the PMPRB's Compliance and Enforcement Policy, a VCU can also be submitted following the issuance of a Notice of Hearing. A VCU submitted at this point must be approved by the Board.
In 2006, three VCUs were approved for
NuvaRing, Organon Canada Ltd. Eloxatin, sanofi-aventis Canada Inc. Hextend, Hospira Healthcare Corporation
In 2007, the Board approved one VCU in the matter of 3M Canada Company and its medicine Airomir.
Nuvaring TM is a new medicine for contraception. It is a flexible, soft, transparent, slow release vaginal ring.
On June 20, 2006, the Vice- Chairperson of the Board accepted a VCU for NuvaRingTM, submitted by Organon Canada Ltd. (Organon).
Organon reduced the average transaction price of NuvaRingTM to a level at or below the 2006 maximum nonexcessive (MNE) price of $13.6791. To offset excess revenues, as calculated by Board Staff, during the period January 17 to June 30, 2005, Organon made a payment to the Government of Canada in the amount of $115,584.93. The remaining excess revenues, for the period July 1, 2005 to June 30, 2006, were offset by the reduction of the price of RemeronRD 15mg, 30mg and 45mg. The price of NuvaRingTM remains under the PMPRB's jurisdiction until the patent expires in 2018.
Eloxatin is used to treat patients with metastatic carcinoma of the colon or rectum whose disease has recurred or progressed during or within 6 months of completion of first-line therapy with the combination of bolus 5-FU/LV and irinotecan.
On July 14, 2006, the Chairperson of the Board accepted a VCU for Eloxatin.
sanofi-aventis Canada Inc. (sanofiaventis) agreed that the MNE prices for Eloxatin 50 mg and 100 mg were $430.9208 and $922.6750 at introduction and that they were $490.5901 and $1,030.0175 in 2006. In lieu of a price reduction for the 50 mg vial and in order to avoid a distortion in the pricing relationship between the 50 mg and 100 mg vials, sanofi-aventis maintained the price of Eloxatin 100 mg vial at $1,000.00 until such time as the MNE price for the 50 mg vial reached $500.00.
In order to offset excess revenues received from the sale of Eloxatin, sanofi-aventis made payments totalling $1,767,078.84 to hospitals, cancer clinics and cancer boards that had previously purchased Eloxatin at excessive prices. The individual payments reflected the distribution of purchases of Eloxatin across Canada up to the end of March 31, 2006.
The price of Eloxatin is under the PMRPB's jurisdiction, at least until the end of the January to June 2019 reporting period.
Hextend is indicated for the treatment of hypovolemia when plasma volume expansion is required.
The Chairperson approved a VCU for Hextend on July 14, 2006.
Hospira Healthcare Corporation (Hospira) agreed that the 2004 and 2005 MNE prices of Hextend were $0.0858 per mL. It will ensure that the average transaction price of Hextend in all future periods does not exceed the MNE price – where the price in the U.S. in local currency terms remains unchanged or increases, the MNE shall be the lower of the CPI-adjusted price and $0.0858 per mL; and where the price in the U.S. in local currency terms decreases, the MNE shall be calculated using the new U.S. price in conducting the International Price Comparison (IPC) test as set out in the Guidelines. Hospira further ensured that the average transaction price for 2006 did not exceed the 2006 MNE price. Hospira offset excess revenues accrued between March 15 and December 31, 2004 by making a payment to the government of Canada in the amount of $8,823.60.
The price of Hextend remains under the PMPRB's jurisdiction until the patent expires in 2014.
Airomir is used for the treatment of asthma, chronic bronchitis, and other breathing disorders.
The Board approved a VCU agreed to by 3M Canada Company (3M Company) and Board Staff, for the payment in full of revenues alleged by Board Staff to have been excessive totalling $485,498.58, derived from January 1, 2004 to December 29, 2006. By order of the Board, the proceeding that was commenced by the issuance of a Notice of Hearing was concluded.
On February 20, 2006, the Board issued a Notice of Hearing pertaining to the allegations of Board Staff that Airomir had been, and was being, sold by 3M Canada at prices exceeding the Excessive Price Guidelines. The Board held a pre-hearing conference on May 19, 2006, and scheduled the hearing to commence on October 19. At the request of 3M Canada, the hearing was postponed. The Board was subsequently informed that 3M Canada sold its marketing rights for Airomir to Graceway Canada Company (Graceway) on December 29, 2006. On May 9, 2007, the Board received a submission for the approval of a VCU to resolve all issues raised by the Notice of Hearing.
Under section 103 of the Patent Act, the Minister of Health may enter into an agreement with the provinces respecting the distribution of the amount collected under the VCU.
For purposes of the application of the Board's Excessive Price Guidelines, Graceway is the Canadian patentee of Airomir as of December 29, 2006. Under the Patented Medicines Regulations, 1994, Graceway is required to file pricing and sales information with the PMPRB twice a year, at regular intervals, as well as file its R&D expenditures annually.
The PMPRB reviews the patentees' prices of patented medicines sold in Canada to ensure that such prices are not excessive and hence protects consumer interests. In the event that the price of a patented medicine appears to be excessive, the Board can hold a public hearing and, if it finds that the price is excessive, it may issue an Order to reduce the patentee's price and to offset the excess revenues.
Since January 2006, the Board has issued eight Notices of Hearing, bringing the total of ongoing hearings to ten. This recent increase in the number of Notices of Hearing may not necessarily represent a longer term trend but is a departure from the previous history of the Board. Indeed, by way of comparison this number is equal to the total of the Notices of Hearing issued by the Board going back to its inception in 1987 through to 2005. Of those previous eight, one hearing was completed; five were resolved through Voluntary Compliance Undertakings, while two others, Dovobet and Nicoderm, are pending.
The reasons for an increase in hearings may involve such factors as the shift in the drug pipeline away from blockbuster new chemicals to more incremental innovations, and, in part, because of notices from third parties about price increases after a period of considerable price stability.
The purpose of these hearings is to determine whether, under sections 83 and 85 of the Patent Act, the patentees are selling or have sold the medicines in question in any market in Canada at prices that, in the Board's opinion, are, or were, excessive, and if so, what order if any, should be made.
| In the matter of |
Indication |
Status |
Adderall XR
Shire BioChem Inc. |
Adderall XR is a medicine indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) |
Final arguments: June 18, 2007
On December 15, 2006, the Board issued a decision (PMPRB-06-D1-ADDERALL XR) on the issue of pre-patent. Shire BioChem had made a motion to the Board for an order that the Board amend its Notice of Hearing to limit the Board's inquiry to the period following the date of issuance of patent 2,348,090, namely, April 13, 2004. The Board dismissed Shire's motion. Shire filed an application for judicial review in this matter with the Federal Court of Canada. The matter has not yet been heard. |
Airomir
3M Canada Company |
Airomir is used for the treatment of asthma, chronic bronchitis, and other breathing disorders. |
VCU approved May 14, 2007 (See page 16 for details) |
Concerta
Janssen-Ortho Inc. |
Concerta is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). |
Hearing: June 11-12, 2007 Closing arguments: August 29, 2007 |
Copaxone
Teva Neuroscience G.P.- S.E.N.C. |
Copaxone 20 mg/1.0 mL syringe is a new formulation of an existing compound (glatiramer acetate) indicated for use in ambulatory patients with Relapsing- Remitting Multiple Sclerosis to reduce the frequency of relapses. |
Closing arguments: June 27, 2007 |
Dovobet
LEO Pharma Inc. |
Dovobet is a dermatological drug administered for bringing psoriasis under control. |
Pending – final resolution |
Nicoderm
Hoechst Marion Roussel Canada Inc. |
Nicoderm is a transdermal nicotine patch, indicated as an aid for smoking cessation for the partial relief of nicotine withdrawal symptoms. |
Pending – final resolution |
Penlac Nail Lacquer
sanofi-aventis Canada Inc. |
Penlac is a new formulation of an existing compound (ciclopirox), indicated as part of a comprehensive nail management program in immunocompetent patients with mild to moderate onychomycosis (due to Trichophyton rubrum) of fingernails and toenails without lunula involvement |
Pre-hearing conference: June 6, 2007 |
Quadracel and Pentacel
sanofi pasteur Limited |
Quadracel is indicated for the primary immunization of infants, at or above the age of 2 months, and as a booster in children up to their 7th birthday against diphtheria, tetanus, whooping cough (pertussis) and poliomyelitis.
Pentacel is indicated for the routine immunization of all children between 2 and 59 months of age against diphtheria, tetanus, whooping cough (pertussis), poliomyelitis and haemophilus influenzae type b disease. It is sold in Canada in the form of a reconstituted product for injection combining one single dose vial of Act HIB (Lyophilized powder for injection) and one single (0.5 mL) dose ampoule of Quadracel (suspension for injection). |
Pre-hearing conference: August 17, 2007 |
Risperdal Consta
Janssen-Ortho Inc. |
Risperdal Consta is a new formulation of an existing compound (risperidone) indicated for the management of the manifestations of schizophrenia and related psychotic disorders. |
Final arguments: June 8, 2007 |
Strattera
Eli Lilly Canada Inc. |
Strattera is for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children 6 years of age and over, adolescents and adults. |
Hearing: dates to be determined |
The Board's hearings are reported on the PMPRB Web site and in its quarterly NEWSletter.
On March 21, 2007, the Federal Court of Canada released its decision and reasons on the application for judicial review filed by LEO Pharma Inc. with regard to the Board's decision in the matter of the price of the patented medicine Dovobet.
The Board issued a Notice of Hearing on November 29, 2004 to hold a public hearing into the price of Dovobet, a dermatological drug administered for psoriasis. Following release of the Board's decision on the merits of this case in April 2006, LEO Pharma filed an application for judicial review with the Federal Court. In its application, LEO Pharma raised several issues, namely the standard of review of the Board's decision, its determination of the appropriate therapeutic class, the application of the International Price Comparison tests, the exclusion of free goods in the calculation of the average transaction price of Dovobet, and allegations of institutional bias with regard to the Board's structure.
In his decision, Justice Blais upheld the Board's decision on all issues except in regards to the exclusion of free goods in the calculation of the average transaction price of Dovobet.
Standard of Review
In his decision, Justice Blais held that, given the nature of the questions raised which were of mixed fact and law, the appropriate standard of review was that of "reasonableness". In the words of Justice Blais: "Moreover, it is important to keep in mind that a decision of the Board on whether or not a medicine is excessively priced is highly discretionary, as the Act (Patent Act) and associated Regulations (Patented Medicines Regulations, 1994) provide very limited guidance on the subject, and thus should be accorded greater deference."7
However, with respect to the allegations of institutional bias, Justice Blais, applying a standard of correctness, upheld the Board's structure. Justice Blais rejected the applicant's argument that the Board lacks sufficient institutional independence and impartiality to provide a patentee with a fair hearing in accordance with the principles of fundamental justice.
Board's Excessive Price Guidelines
In his decision, Justice Blais acknowledged that section 85 of the Act is drafted in very general terms, thereby giving the Board broad discretion to determine excessive pricing issues. Justice Blais also acknowledged that it is appropriate for the Board to look to its Guidelines for rationale and methodology in considering the application of the factors in subsection 85(1), provided the Board does not consider itself bound by those Guidelines.
In considering the issue of the application of the appropriate price tests, Justice Blais upheld the Board's decision that the price of Dovobet ought to be no higher than the combined prices of its two constituent elements (Dovonex and Diprosone).
Free Dovobet
With respect to the exclusion of the free goods in the calculation of the average transaction price (ATP) of Dovobet, Justice Blais ruled that the distribution of free medicine must be included because the Patented Medicines Regulations, 1994 require the patentee to report the price at which it has sold a patented medicine taking into consideration "any reduction [in price] given as a promotion or in the form of rebates, discounts, refunds, free goods, free services, gifts or any other benefits of a like nature." (emphasis added)
7 Federal Court of Canada, http://decisions.fct-cf.gc.ca/en/2007/2007fc306/2007fc306.html, page 8, para 15.
- Trends in Sales of Patented Drugs
- Price Trends
- Comparison of Foreign Prices to Canadian Prices
- Utilization of Patented Drugs
- Manufacturing Trends in Canada
- Canadian Sales in The Global Context
- Analysis of Research and development Expenditure
Trends in Sales of Patented Drugs8
Patentees are required, under the Patented Medicines Regulations, 1994, (Regulations) to submit detailed information on their sales of currently patented drugs, including information on quantities sold and net revenues received by product, class of customer and province/territory. This information allows the PMPRB to analyze trends in sales, utilization and prices among patented drugs. Results of this analysis are presented in this section.9
Sales and Prices
Canadians spend much more today on drugs than they did a decade ago. However, it is important to understand that increased spending on drugs does not, in itself, imply rising drug prices. Previous Annual Reports have found little or no change in patented drug prices along with sales growth of 10-20%. In these instances sales growth was driven by changes in the volume and composition of drug utilization.10 A variety of factors can produce such changes. These include:
- increases in total population;
- changes in the demographic composition of the population (e.g., shifts in the age-distribution toward older persons with more health problems);
- increased incidence of health problems requiring drug therapy;
- changes in the prescribing habits of physicians (e.g., shifts away from older, less expensive drugs to newer, more expensive medications);
- greater use of drug therapy instead of other forms of treatment; and,
- use of new drug products to treat conditions for which no effective treatment existed previously.
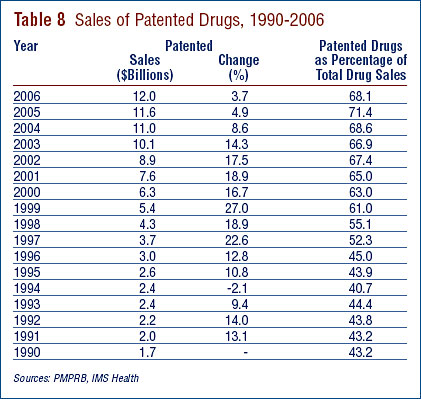
Sales Trends9
Table 8 reports patentees' total sales of patented drugs in Canada for the years 1990 through 2006. Sales of patented drugs rose to $12.0 billion from $11.5 billion in 2005, an increase of 3.7%. By comparison, annual growth in sales of patented drugs stood at 27.0% in 1999 and remained in double-digits until 2003. Sales growth declined to 8.6 % in 2004, and declined again to 4.9 % in 2005.
The third column of Table 8 presents sales of patented drugs as a share of overall drug sales.11 Since 1994 patented drug sales as a share of total drug sales have increased from approximately 40% to over 68% in 2006.
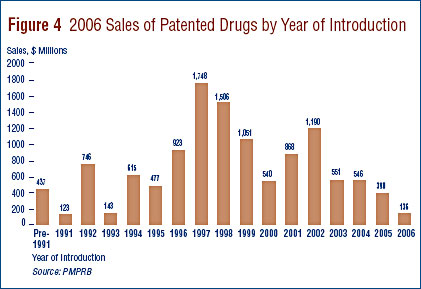
PMPRB annual report 2006 The pronounced decline in sales growth of the last few years is a striking development. Last year's Annual Report argued that throughout the 1990s, sales growth was largely driven by a succession of new "blockbuster" products that ultimately achieved very high sales volumes, and that since the beginning of the current decade, the pharmaceutical industry had not introduced new high-volume products in sufficient numbers to sustain the double-digit sales growth seen in the 1990s. As a result, 2005 sales of patented drugs were still dominated by products introduced between 1995 and 1999.
These patterns appear once again in 2006 sales. Figure 4, on page 22, breaks down patentees' 2006 sales by the year in which products were first sold in Canada. The results in Figure 4 clearly demonstrate that sales of patented drugs are still dominated by products introduced in the second half of the 1990s: in 2006, products introduced in the seven years from 2000 to 2006 accounted for sales of $4.2 billion, compared to $5.7 billion for products introduced in the five years from 1995 to 1999. The latter amount represents nearly half of 2006 sales.
Sales by Therapeutic Class
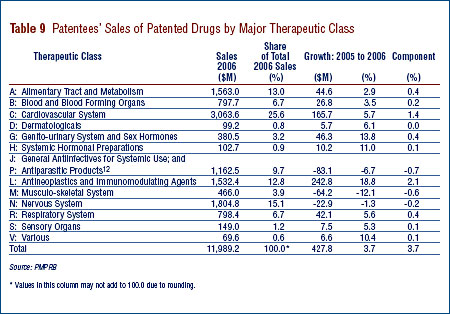
The PMPRB normally classifies drugs using the World Health Organization's (WHO) Anatomical Therapeutic Chemical (ATC) classification system. This is a hierarchical system that classifies drugs according to their principle therapeutic use and chemical composition. At its most aggregate level, "ATC Level 1", the ATC system classifies drugs according to the aspect of human anatomy with which they are primarily associated.
Table 9 on page 23 breaks out sales of patented drugs in Canada in 2006 by major therapeutic class, defined by ATC Level 1. The Table gives the 2006 sales for each class, the share of the total this represents and the rate at which sales grew relative to 2005. The last column multiplies each class' rate of sales growth by its share of overall sales: each resulting entry represents the component of overall sales growth attributable to drugs in the corresponding therapeutic class. By this measure, the primary drivers of 2005-to-2006 sales growth were:
- antineoplastics and immunomodulating agents; and
- drugs related to the cardiovascular system (such as lipid-reducing agents and drugs treating hypertension).
These two classes accounted for 2.1 percentage points and 1.4 percentage points of sales growth, respectively. This is the third consecutive year antineoplastics and immunomodulating agents have emerged as the leading contributor to sales growth. Cardiovascular drugs have been a leading driver of sales growth for many years. It is worth noting that several therapeutic classes that have been important sources of sales growth in past years – most notably drugs related to the alimentary tract and the nervous system – contributed relatively little to sales growth this year.
8 Throughout this chapter the term a "patented drug" denotes any product currently subject to PMPRB price review.
9 All statistical results for 2006 presented in this Annual Report are based on sales data submitted to the PMPRB as of the end of 2006.
On occasion, patentees report revisions to previously submitted data, which revisions can appreciably impact the statistical values presented in this Report. To account for this, the PMPRB has adopted the practice of reporting recalculated sales figures (Trends in Sales of Patented Drugs), price/quantity indices (Price Trends, page 24) and Foreign-to-Canadian price ratios (Comparison of Foreign Prices to Canadian Prices, page 28) for the five years preceding the current Annual Report year. All such recalculated values reflect currently available data. As a result, where data revisions have occurred, values reported here may differ from those presented in earlier Annual Reports.
10 Studies conducted by the PMPRB of public pharmaceutical insurance plans indicate that increased utilization of existing and new drugs accounts for most of the recent growth in expenditures. PMPRB, Provincial Drug Plan Overview Report: Pharmaceutical Trends, 1995/96 -1999/00, September 2001.
11 The denominator in this ratio, total drug sales, comprises sales of patented drugs, generic drugs and non-patented branded drugs. The estimate of total drug sales used to calculate the 2006 ratio is based on data provided in IMS Health's Canadian Pharmaceutical Market: Drug Store and Hospital Purchases, December, 2006. In previous years, IMS data was used to calculate generic sales only, while sales of non-patented branded products were estimated from data submitted by patentees. Because of anomalies in this latter estimate arising from year-to-year changes in the set of patentees, the PMPRB now uses IMS data to directly estimate total drug sales.
sup>12 These groups have been combined for reasons of confidentiality.
The PMPRB uses the Patented Medicine Price Index (PMPI) to monitor trends in prices of patented drugs. The PMPI is a price index measuring the average year-over-year change in the ex-factory prices of patented drugs sold in Canada. The index is constructed using a chained Laspeyres price index formula, taking a sales-weighted average of price changes at the level of individual drugs.13 This is similar to the approach Statistics Canada uses to construct the Consumer Price Index (CPI). The PMPI is updated every six months using price and sales information submitted by patentees.14 It encompasses only prices of patented drugs intended for human use.15
It is important to understand the conceptual relationship between the PMPI and drug costs. The PMPI does not measure changes in the utilization of patented drugs: a quantity index, the PMQI, is calculated for this purpose (see Utilization of Patented Drugs on page 32). The PMPI does not measure the cost-impact of changes in prescribing patterns or the introduction of new medicines. By design, the PMPI isolates the component of sales growth attributable to changes in the prices of patented drugs.
Figure 5 provides year-over-year changes in the PMPI for the years 1988 through 2006. As measured by the PMPI, prices of patented drugs declined on average by 0.2% from 2005 to 2006. This small decline in the PMPI in 2006 follows two years of appreciable increases.
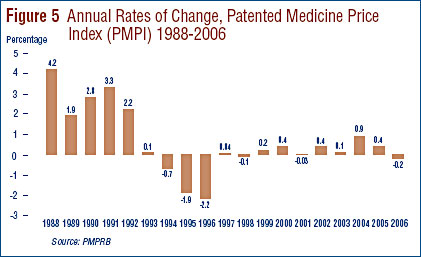
Comparison of PMPI and CPI
The Patent Act (Act) provides that, among other factors, the PMPRB shall consider changes in the Consumer Price Index (CPI) in determining whether the price of a patented drug is excessive. Figure 6 plots year-over-year rates of change in the PMPI against corresponding changes in the CPI. Inflation, as measured by the CPI, has exceeded the average increase in patented drug prices almost every year since 1988.16 This pattern continued in 2006, with the CPI rising by 2.0%17 while the PMPI declined by 0.2%.
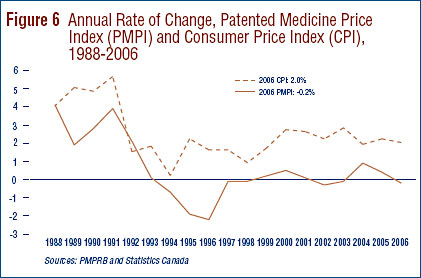
That the PMPI has not kept pace with the CPI is not surprising. The Board's Excessive Price Guidelines (Guidelines) allow the price of a patented drug to rise by no more than the CPI over any three-year period. (The Guidelines also impose a cap on year-over-year price increases equal to one-and-one-half times the current year rate of CPIinflation.) This effectively establishes CPI-inflation as an upper bound on the rate at which the PMPI may rise over any period of three years.18 Increases in the PMPI normally do not reach this upper bound because many patentees do not raise their prices by the full amount permitted under the Guidelines or reduce their prices.
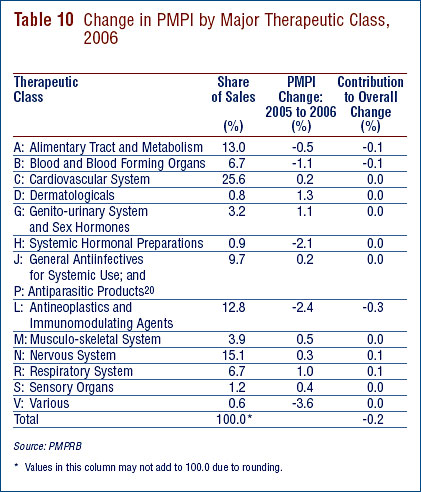
Price Change by Therapeutic Class
Table 10 provides average rates of price change among patented drugs at the level of major therapeutic classes. Results in this Table were obtained by applying the PMPI methodology to data segregated by their ATC Level I class. The Table lists the share of each therapeutic class in total sales of patented drugs, as well as the average percentage price change among the drugs in each class. The last column multiplies the rate of price change in each class by its share of overall sales: this yields an approximate decomposition of overall PMPI change, each entry in the column representing the component of overall PMPI change attributable to drugs in the corresponding therapeutic class. By this measure, antineoplastics and immunomodulating agents were the largest contributors to overall price change in 2006.19 Note that in all therapeutic classes average rates of price change were well below CPI inflation.
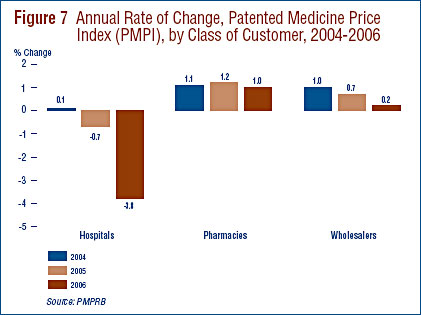
Price Change by Class of Customer
Figure 7 presents average rates of price change by class of customer.21 These results were obtained by applying the PMPI methodology to data on sales of patented drugs made specifically to hospitals, to pharmacies and to wholesalers.22 Rates of 2005-to-2006 price change ranged from 1.0% for direct sales to pharmacies to -3.8% for sales to hospitals. Not surprisingly, the rate of price change for sales to wholesalers (which accounts for about threequarters of all sales) is closest to the overall change in the PMPI. Note that in all customer classes, rates of price change were substantially less than CPI-inflation.
It is clear from Figure 7 that the slight decline in the overall PMPI was the result of falling prices paid by hospitals: a PMPI covering sales to pharmacies and wholesalers would have risen by approximately 0.4% between 2005 and 2006.
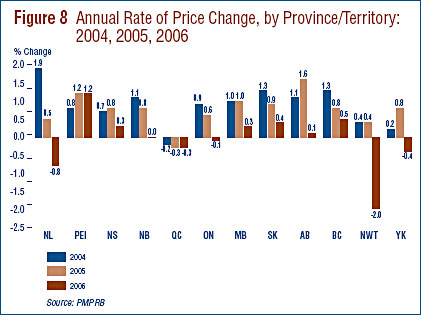
Price Change by Province/Territory
Figure 8 presents average rates of price change by province/territory. These results were obtained by applying the PMPI methodology to data segregated by the province/ territory in which the sale took place. Rates of price change range from 1.2% in Prince Edward Island to -2.0% in the Northwest Territories. Average price increases in six of the thirteen provincial/territorial jurisdictions were offset by the modest declines in Ontario and Quebec, resulting in the average national price decrease of 0.2%. Note that in all jurisdictions, average rates of price change were well below CPI-inflation.
Price Change by Country
In accordance with the Act and the Regulations, patentees must report publicly available ex-factory prices of patented drugs in seven foreign countries. These countries are: France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States. The PMPRB uses this information to:
- conduct the international price comparison tests specified in the Guidelines; and
- compare the Canadian prices of patented drugs to those in other countries.
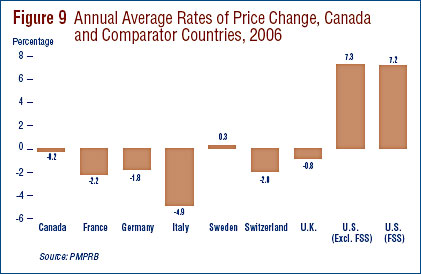
Figure 9 gives annual 2005-to-2006 rates of price change for Canada and each of the seven comparator countries. These results were obtained by applying the PMPI methodology (with weights based on Canadian sales patterns) to international price data submitted to the PMPRB. Note that two results are presented for the U.S.: the first of these is restricted to US prices reported by patentees, the second incorporates prices from the U.S. Federal Supply Schedule (FSS) in addition to reported prices.23
Five of seven comparator countries registered overall price declines in 2006, the exceptions being the U.S. and Sweden. Of these five, Italy saw the largest average decline (-4.9%). In contrast, US prices rose by more than 7% on average.
13 More exactly, at the level defined by Health Canada's Drug Identification Number (DIN).
14 Annualized PMPI results are obtained by averaging results for the first and last six months of each year.
15 See the PMPRB's A description of the Laspeyres methodology used to construct the Patented Medicine Price Index (PMPI), June 2000, for a detailed explanation of the PMPI. Restricting the PMPI to products for human use began in 1999.
16 1992 is the only year in which the PMPI rose at a faster rate than the CPI. To facilitate and encourage compliance by patentees, the PMPRB's CPI-adjusted methodology uses the forecast rate of CPI inflation published by the Department of Finance. The forecast CPI-inflation rate for 1992 had been 3.2%, but the actual rate was 1.5%. For a full explanation of the CPI-adjusted methodology, please refer to Schedule 4 of the PMPRB's Compendium of Guidelines, Policies and Procedures, available on the PMPRB Web site under Legislation, Regulations, Guidelines.
17 Statistics Canada, CANSIM, Series V735319.
18 In theory the one-year increase cap allows the year-over-year rise in the PMPI to exceed CPIinflation. This has not occurred since the PMPRB instituted its current CPI methodology.
19 Suppose R represents the overall rate of change in the PMPI. Suppose there are N therapeutic classes, indexed by 1, 2 … N. Let R(i) represent the average rate of price change in major therapeutic class i obtained by means of the PMPI methodology. Using the fact that R is a sales-weighted average of price changes taken over all patented drugs, it is easy to derive the following relationship:
R = w(1)R(1) + w(2)R(2) + … + w(N)R(N),
where w(i) represents the share of therapeutic class i in the sales of patented drugs. This relationship provides the basis for the decomposition in the last column of Table 10. Each term on its right-hand-side multiplies the average rate of price change for a given therapeutic class by its share of overall sales. The resulting value is readily interpreted as the corresponding class' contribution to the change in the overall PMPI. Note that the size of this contribution depends on both the rate of price change specific to the class and its relative importance (measured by its share of sales).
As noted in the text, the decomposition in Table 10 is approximate. This is because the weights used for this purpose are derived from annual sales data, whereas PMPI is calculated from data covering periods of six months.
20 These groups have been combined for reasons of confidentiality.
21 The Patented Medicines Regulations, 1994 require patentees to file information according to 4 classes of customers: Wholesalers, Hospitals, Pharmacies, Others.
22 Results for a fourth customer class, "Others", are not provided. Buyers in the "Others" class are principally healthcare institutions other than hospitals, such as clinics and nursing homes.
23 The pharmaceutical industry in the U.S. has argued that the publicly available prices in that country do not reflect actual prices because of confidential discounts and rebates. Effective January 2000, and following public consultation, the PMPRB began including prices listed in the U.S. Federal Supply Schedule (FSS) in calculating the average US price of patented drugs. The FSS prices are negotiated between manufacturers and the U.S. Department of Veterans' Affairs. They are typically less than other publicly available US prices reported to the PMPRB by patentees
Tables 11 and 12 provide detailed statistics comparing the foreign prices of patented drugs to their Canadian prices. Each table provides four sets of average price ratios. These are differentiated according to (1) the averaging formula applied and (2) the method by which foreign prices were converted to Canadian dollar equivalents. The Tables also show the number of drugs (DINs) and the volume of sales encompassed by each reported statistic.24
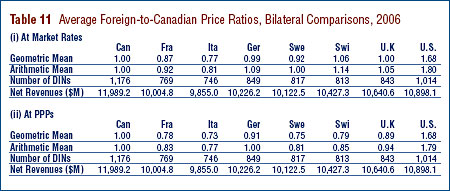
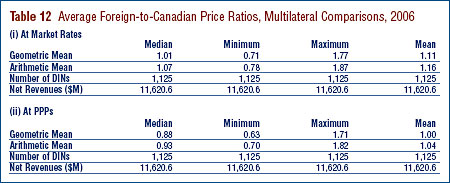
The PMPRB has traditionally reported average Foreign-to-Canadian price ratios constructed as a sales-weighted geometric mean of individual ratios. Such results are included in Tables 11 and 12 (under the label "Geometric Mean"). The Tables also provide results obtained using a salesweighted arithmetic average (under the label "Arithmetic Mean").25 These statistics provide an exact answer to questions of the type:
"How much more/less would Canadians have paid for the patented drugs they purchased in 2006 if they had paid Country X prices rather than Canadian prices?"
For example, Table 11 states that the 2006 average French-to-Canadian price ratio obtained using the arithmetic mean is 0.92. This means Canadians would have paid 8% less for the patented drugs they purchased in 2006 had they been able to buy these products at French prices.
For many years the PMPRB has reported average Foreign-to-Canadian price ratios with foreign prices converted to their Canadian dollar equivalents by means of market exchange rates (or, more exactly, the 36-month moving-averages of market rates the PMPRB normally uses in applying the Guidelines). Last year, the PMPRB began reporting Foreignto- Canadian price ratios with currency-conversion at purchasing power parity (PPP). The PPP between any two countries measures their relative cost-of-living expressed in their own currencies. In practice, cost-of-living is determined by pricingout a standard set (or "basket") of goods and services at prices prevailing in each country. Because PPPs are designed to represent relative costof- living, they offer a simple way to account for differences in national price levels when comparing individual prices, incomes and other monetary values across countries. When applied in calculating average foreignto- Canadian price ratios, they produce statistics allowing us to answer questions of the form:
"How much more/less consumption of other goods and services would Canadians have sacrificed for the patented drugs they purchased in 2006 had they lived in Country X?"
Questions of this type cannot be answered by simply comparing drug prices. Rather, one must first calculate what each price represents in terms of goods and services foregone. PPPs are designed for such purposes.
Bilateral Comparisons
Table 11 is devoted to bilateral comparisons of prices in each of the seven comparator countries to corresponding Canadian prices. Focusing on the results obtained with currency conversion at market exchange rates (and calculated as a geometric mean), it appears that Canada is in the middle of the pack with regard to the prices of patented drugs. Prices in France and Italy are, on average, appreciably less than Canadian prices, while prices in Switzerland and the U.S. are higher. As in previous years, US prices were substantially higher than prices in Canada or any other comparator country.
Figure 10 puts these results in historical perspective. In 1987, Canadian prices were, on average, below US prices but above those in all other countries. By the mid-1990s, the situation had changed dramatically, with Canadian prices in the mid-range of the six European countries.
It should be note that the average price ratios obtained with currency conversion at PPPs tell a very different story. Once one accounts for international differences in cost-ofliving, Canada emerges as a relatively high-cost country. It appears Canadians incurred a greater consumption-cost for the patented drugs they purchased in 2006 than did residents of every comparator country other than the U.S. The case of Switzerland is instructive in this respect. In purely monetary terms, the prices of patented drugs in Switzerland are appreciably higher than corresponding Canadian prices. Despite this, residents of Switzerland sacrifice less in real terms – that is, in terms of foregone consumption – to acquire patented drugs than Canadians, because of Switzerland's very high cost of living.
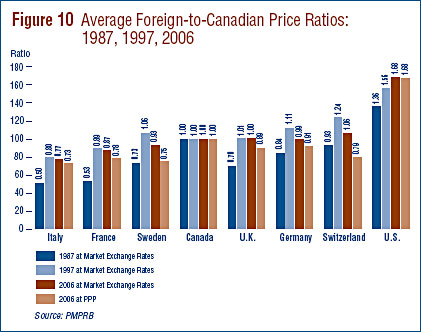
Multilateral Price Comparisons 26
Table 12 gives the average Foreignto- Canadian price ratios for several multilateral measures of foreign price levels. First among these is the "median international price" (MIP), calculated from prices observed in the seven comparator countries. Other average price ratios compare the minimum, maximum and simple mean of foreign prices to their Canadian counterparts.

Focusing again on results at market exchange rates (and calculated as a geometric mean), the average MIP-to- Canadian price ratio stood at 1.01 in 2006, representing a substantial decline from the value of 1.09 reported last year. Figure 11 puts this result in historical perspective. MIPs were on average 19% below Canadian prices in 1987 (or, conversely, Canadian prices were 23% above the MIP). By 1998, MIPs were on average 14% higher than Canadian prices. The average MIP-to-Canadian price ratio has remained above parity throughout this decade. That is, prices in Canada have been below the MIP.
Results obtained with other multilateral measures are much as one would expect. Interestingly, it appears mean foreign prices typically produce higher Foreign-to-Canadian price ratios than do MIPs. This is readily explained by the influence of US prices: typically much higher than prices elsewhere, US prices nearly always figure in the calculation of the mean foreign price but seldom serve as median international prices.
As with the bilateral comparisons, differences between results obtained at market exchange rates and at PPPs are striking. These affirm the idea that while Canada may be a "mediumprice" country in purely monetary terms, Canadians actually sacrifice appreciably more consumption to acquire patented drugs than do residents of most comparator countries. With currency conversion at PPPs, the average MIP-to-Canadian price ratio (calculated as a geometric mean) was 0.88 in 2006, substantially less than the value of 1.01 obtained at market exchange rates.
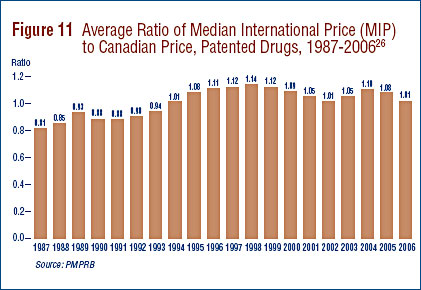
Figure 12 offers more detail on the product-level MIP-to-Canadian ratios underlying the reported averages. This figure distributes 2006 sales of every patented drug according to the value of its MIP-to-Canadian price ratio (or, more exactly, according to the range into which that ratio fell). The figure indicates that in 2006 product-level price ratios were heavily concentrated around parity: cases where the MIP-to-Canadian price ratio was between 0.75 and 1.25 accounted for 71.2% of Canadian sales. Instances where the MIP was less than 75% of the Canadian price accounted for 9.5% of sales. Instances where the MIP exceeded the Canadian price by more than 25% accounted for 19.3% of sales.
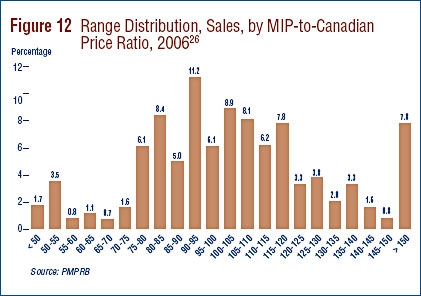
Average Price Ratios: Analysis of Changes
Recall that Figure 11, on page 31, indicates that a substantial decline in the average MIP-to-Canadian price ratio occurred between 2005 and 2006. Given the construction of the average ratio, there are four factors that might account for the observed decline:
- appreciation of the Canadian dollar against other currencies;
- declining foreign prices;
- rising Canadian prices; and
- a shifting in sales-weights favouring drugs with relatively low MIP-to-Canadian price ratios.
Further data analysis reveals that the observed decline in the average MIPto- Canadian price ratio is the result of recent appreciation of the Canadian dollar against other currencies and, to a lesser extent, changes in foreign prices. Using 2005 exchange rates instead of their 2006 values yields a 2006 MIP-to-Canadian average price ratio some five points higher than the 1.01 reported in Table 12 on page 29. Using 2005 foreign prices instead of their 2006 values adds another 1-2 points to the 2006 average price ratio. On the other hand – and as might be expected from the fact that the overall PMPI was nearly constant between 2005 and 2006 – changes in Canadian prices appear to have had practically no impact on the average MIP-to-Canadian price ratio.
24 The number of drugs and sales encompassed vary from comparator to comparator because it is not always possible to find a matching foreign price for every patented drug product sold in Canada. It is worth noting in this regard that all of the average price ratios reported in Tables 11 and 12, on page 29, cover at least 82% of 2006 Canadian sales. The reported US-to-Canada price ratios cover about 91% of 2006 sales.
25 Let RG represent the average price ratio obtained using the geometric method, RA the average price ratio obtained using the arithmetic. Let p(i) represent the Canadian price of drug i, pf(i) its foreign price (converted to Canadian dollars) and w(i) its share of Canadian sales. Then RG = Ð [pf(i)/p(i)]w(i) (where Ð signifies multiplication over all patented drugs), while RA = Ów(i)[pf(i)/p(i)] (where Ó signifies summation over all patented drugs).
It is readily demonstrated that RG can never exceed RA. It is also possible to show that the difference between RA and RG will increase with the extent of variation among individual price ratios, and that RG will equal RA only in the special case where all price ratios have the same value.
26 Note that in previous Annual Reports, the PMPRB has undertaken multilateral price comparisons by means of Canadian-to-Foreign ratios. For the sake of consistency (with the approach taken in the case of bilateral comparisons), the Annual Report will henceforth use Foreign-to-Canadian ratios for this purpose.
The price and sales data used to calculate the PMPI also allow the PMPRB to examine trends in the quantities of patented drugs sold in Canada. The PMPRB maintains the Patented Medicine Quantity Index (PMQI) for this purpose.27 Figure 13 displays average rates of utilization growth, as measured by the PMQI, from 1988 through 2006. These results confirm that growth in the utilization of patented drugs has been the primary source of rising sales, with rates of utilization growth roughly tracking rates of sales growth in recent years.28 This pattern continued in 2006, with utilization of patented drugs growing by 5.4%.
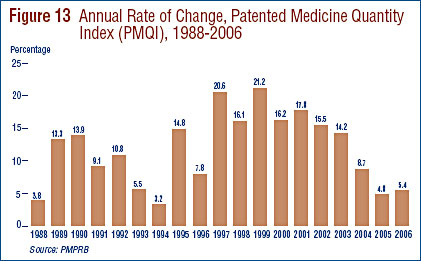
Utilization Growth by Therapeutic Class
Table 13 provides average rates of utilization growth among patented drugs at the level of major therapeutic classes. The results in this table were obtained by applying the PMQI methodology to data segregated by ATC Level I class. As in Table 10, on page 26, the last column multiplies the rate of quantity change for each class by its share of overall sales, yielding an approximate decomposition of overall PMQI change into contributions attributable to each therapeutic class. The largest entries in this column identify the primary drivers of quantity change.29 By this measure the primary drivers of utilization growth in 2006 were:
- antineoplastics and immunomodulating agents; and
- drugs related to the cardiovascular system.
These to classes accounted for about two-thirds of the overall change in utilization indicated by the PMQI. Utilization of drugs related to the musculo-skeletal system declined substantially (-11.2 %), which reduced the PMQI by 0.4 %.
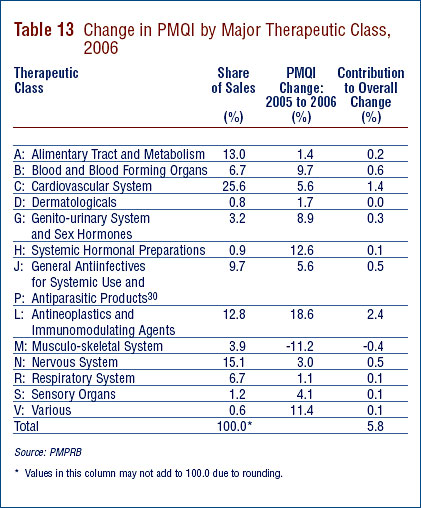
27 Like the PMPI, the PMQI is calculated using a chained Laspeyres index formula, with ratios of physical quantities in successive periods replacing the price ratios of the PMPI. Here again, the aggregate value of the index is obtained as a revenue-weighted average of ratios at the level of individual products. Since the PMQI covers only patented drugs it should not be taken to represent utilization trends in the overall pharmaceutical market.
28 Under normal conditions, the annual rates of change in the PMPI and PMQI will sum to a value approximating the rate of change in patent drug sales. The algebraic relationship is not exact due to (1) interactions of price and quantity changes, (2) the positive impact on sales of new patented drugs, and (3) the negative impact on sales of patent expiries and product withdrawals. This last effect seems to have been unusually large in 2006: this explains why sales rose by only 3.7% between 2005 and 2006, while utilization (as measured by the PMQI) rose on average by 5.4%.
29 As in the case of Table 10, on page 26, this decomposition of PMQI change is only approximate, because it is based on annual sales figures, while the PMQI is calculated from sales data covering periods of six months.
30 These groups have been combined for reasons of confidentiality.
The global drug industry is dominated by a number of large multinational enterprises based in countries other than Canada. Most of these companies have Canadian subsidiaries which, along with a few Canadianbased manufacturers, account for the manufacture, sale and distribution of drugs in Canada.
According to Statistics Canada, shipments by Canadian drug manufacturers amounted to $7.9 billion in 2006, accounting for 1.3 % of total shipments in the manufacturing sector.31 The sector employed 29,375 persons, accounting for 1.6 % of total employment in manufacturing. 32
Figure 14 provides year-over-year rates of change in total shipments and employment in drug manufacturing.
31 Since the publication of the PMPRB Annual Report 2005, Statistics Canada has rebenched the manufacturing shipments data from the 2002 Annual Survey of Manufacturers to the 2004 Annual Survey of Manufacturing. The rebenching process recast pharmaceutical and medicine manufacturing shipments significantly below previous estimates.
32 Statistics Canada, CANSIM, Series V800188 and V1709627
 IMS Health regularly reports on patentees' sales to the retail sector across a wide range of countries. IMS reports that in 2006 such sales amounted to $440.3 billion among major markets.33 Figure 15 shows how this amount was distributed among these markets. Drug sales in Canada accounted for 3.5 of total major-market sales. The US market is by far the largest, with drug sales exceeding the combined sales of all other major markets.
IMS Health regularly reports on patentees' sales to the retail sector across a wide range of countries. IMS reports that in 2006 such sales amounted to $440.3 billion among major markets.33 Figure 15 shows how this amount was distributed among these markets. Drug sales in Canada accounted for 3.5 of total major-market sales. The US market is by far the largest, with drug sales exceeding the combined sales of all other major markets.
Figure 16 gives Canada's share of major-market sales for each of the years 2001 through 2006.34 This share has risen 2.4% in 2001 to 3.5% in 2006.
Figure 17 compares sales growth in Canada to that in other major markets. In recent years, Canadian pharmaceutical sales have risen at a faster rate than elsewhere. This pattern continued in 2006, with year-over-year sales growth in Canada (7%)35 ahead of growth in other major markets (5%).
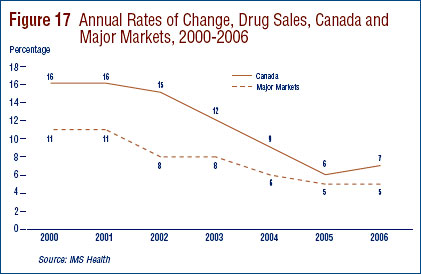
Figure 18 gives rates of 2005-to- 2006 sales growth for individual major markets. Based on IMS data, Canadian sales growth matched that in the U.S. at 7% and exceeded growth observed in all other comparator countries.
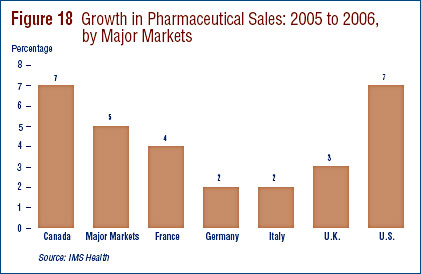
The proportion of national income allocated to the purchase of pharmaceuticals provides another way to compare drug costs across countries.36 Figure 19 gives drug expenditures as a share of Gross Domestic Product (GDP) in Canada and the seven comparator countries, based on data for 2004. Drug expenditures absorbed between 1.1% and 2.0% of GDP in the seven comparator countries.
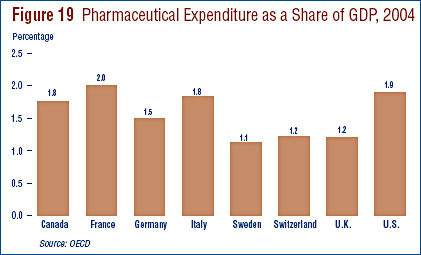
The share of national income absorbed by pharmaceutical expenditures has risen in most developed countries in recent years. Table 14 shows that, except for Sweden, pharmaceutical expenditures grew faster than GDP between 2000 and 2004 in Canada and each of the comparator countries. The results for Canada and the U.S. are especially striking: in both countries pharmaceutical expenditure grew at roughly twice the rate of national income.
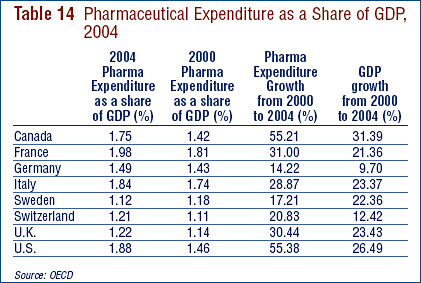
Composition of Expenditures
Table 15 gives the composition of patentees' sales by therapeutic class across countries.37 With only a few exceptions, these results imply a remarkable degree of uniformity across countries. In all countries, sales are dominated by cardiovascular and central nervous system products, which account for 37% to 47% in all cases. The next two leading classes – products treating gastrointestinal and respiratory problems – account for a further 22% to 28% of sales.
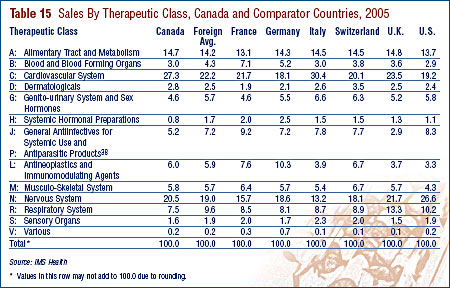
33 IMS Health's Retail Drug Monitor, 2006 (www.imshealth.com). IMS Retail Drug Monitor provides estimates of direct (i.e., from the manufacturing company) and indirect (i.e., through a wholesaler) drug purchases by pharmacies in 13 major markets: Argentina, Australia, Brazil, Canada, France, Germany, Italy, Japan, Mexico, New Zealand, Spain, the U.K. and the U.S. These figures are at ex-manufacturer prices and include all prescription and certain over-the-counter data. IMS estimates the above 13 markets account for over two thirds of the world pharmaceutical market. This implies Canada's share of the total world market is approximately 2.5%.
34 To calculate the shares given in Figures 15 and 16, it is necessary to first express national sales data in a common currency. IMS Health uses market exchange rates for this purpose. This means the Canadian shares reported here can be strongly influenced by changes in relative value of the Canadian dollar.
35 This Canadian growth rate reported here differs from that reported in Table 8, on page 21, for a number of reasons. Most importantly, it is derived from sales data encompassing non-patented as well as patented drugs. Note as well that these data cover only sales to the pharmacy sector.
36 Comparisons made on this basis will reflect international differences in prices, in overall utilization in patterns of therapeutic choice, as well as differences in national income.
37 The data used here cover only sales to pharmacies.
38 These groups have been combined for reasons of confidentiality.
With the adoption of the 1987 amendments to the Patent Act (Act), Canada's Research Based Pharmaceutical Companies (Rx&D) made a public commitment that brand name manufacturers would increase their annual research-anddevelopment (R&D) expenditure to 10% of sales revenue by 1996.39
This chapter provides key statistics on the current state of pharmaceutical research investment in Canada. Under the Act, the PMPRB monitors and reports on R&D spending, but has no regulatory authority over the amount or type of research spending by patentees.
The Act requires each patentee to report its revenue from sales of drugs (including revenue from sales of non-patented drugs and from licensing agreements) and R&D expenditure in Canada related to medicines. The Patented Medicines Regulations, 1994 (Regulations) require that R&D data submitted to the PMPRB be accompanied by a certificate affirming the submitted information is "true and correct". The Board does not audit submissions, but it does review submitted data for anomalies and inconsistencies, seeking corrections or clarifications from patentees where these are detected. To confirm that Board Staff has correctly interpreted submitted data, each patentee is given the opportunity to review and confirm the accuracy of its own R&D-to-sales ratio before publication of this report.
Companies without sales of patented medicines need not report on R&D expenditure. As new patents are granted and existing patents expire, the set of companies required to file R&D data may change from year to year.
For 2006, a total of 72 companies selling drug products for human and veterinary use filed reports on their R&D expenditure.40 Of these, 28 companies were members of Rx&D.
Sales Revenue
For reporting purposes, sales revenue is defined as all revenue from Canadian sales of medicines41 and from licensing agreements.
As shown in Table 16, patentees reported total sales revenue of $14.9 billion from Canadian sales of drugs in 2006, an increase of 4.7% over 2005. Sales revenue reported by Rx&D members totalled $11.1 billion, accounting for 75% of the total. Less than 1% of reported sales revenue was generated by licensing agreements.
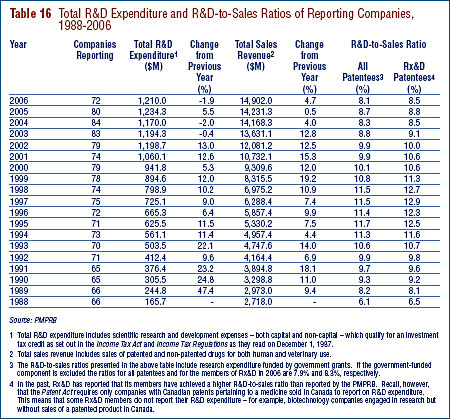
R&D Expenditure
Pursuant to Section 6 of the Regulations, patentees are required to report R&D expenditure that would have been eligible for an Investment Tax Credit for scientific research and experimental development under the provisions of the Income Tax Act in effect on December 1, 1987. By this definition, R&D expenditure may include current expenditure, capital equipment costs and allowable depreciation expenses. Market research, sales promotions, quality control or routine testing of materials, devices or products and routine data collection are among the types of expenditure not eligible for an investment tax credit, and are not to be included in patentees' filings.
As shown in Table 16, total 2006 R&D expenditure reported by patentees was $1,210 million, a decrease of 1.9% over 2005. Rx&D members reported R&D expenditure of $949 million in 2006, accounting for 78.4% of all reported expenditure. This represents a decline of 8.7% relative to 2005 Rx&D expenditure. By comparison, non-Rx&D members reported R&D expenditure of $261 million in 2006, an increase of 34.5% over the corresponding figure for 2005.
R&D-to-Sales Ratios
The ratio of R&D expenditure to sales revenue among all patentees was 8.1% in 2006, down from 8.7% in 2005. The ratio for members of Rx&D was 8.5%, down from 8.8% in the previous year. Figure 20 shows that R&D-to-sales ratios have declined markedly in recent years, after reaching a maximum in the late 1990s. This is the sixth consecutive year the overall ratio has fallen below 10% and the fourth year in which the Rx&D ratio has failed to achieve this target value.
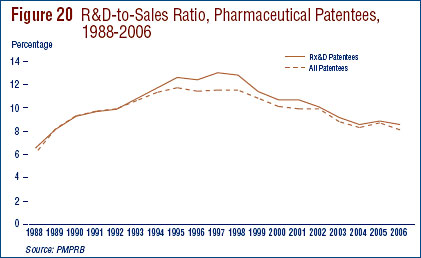
Table 21 in Annex 3, on page 58, provides details on the range of R&D-to-sales ratios. Of the 72 companies reporting in 2006, 56 (77.7%) had R&D-to-sales ratios of 10% or less in 2006. These companies accounted for 68.1% of total sales revenue. Table 22 in Annex 3, on page 59, lists all reporting patentees and their R&D-to-sales ratios.
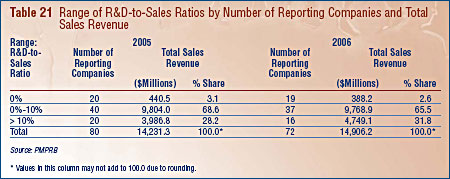
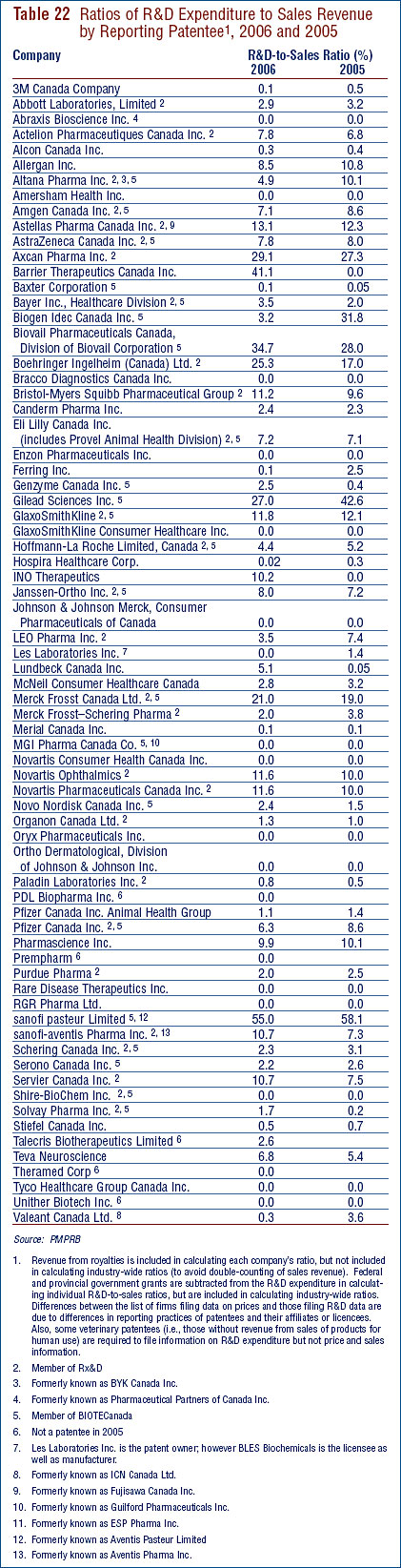
Current Expenditure by Type of Expenditure 42
Current R&D expenditure was $1,159 million in 2006, accounting for 95.8% of total R&D expenditure. Capital equipment costs and allowable depreciation expenses made up 2.4% and 1.8% of total R&D expenditure, respectively.
Current Expenditure by Type of Research
Table 17 and Figure 21, on page 41, give the allocation of 2006 current expenditure among basic research, applied and other qualifying R&D. Basic research is defined as work that advances scientific knowledge without a specific application in view. Patentees reported spending $232.4 million on basic research, representing 20% of current R&D expenditure. Basic research increased by 8% in 2006 relative to the previous year.
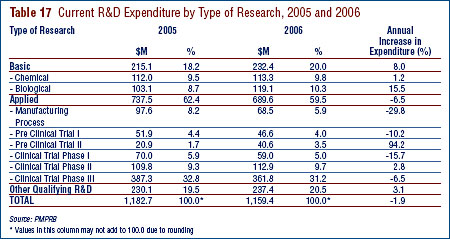
Applied research is directed toward a specific practical application, comprising research intended to improve manufacturing processes, pre-clinical trials and clinical trials. Patentees reported spending $689.6 million on applied research, representing 59.5% of current R&D expenditure. Clinical trials accounted for 77.3% of applied research expenditure.
Other qualifying research (includes drug regulation submissions, bioavailability studies and Phase IV clinical trials) accounted for the remaining 20.5% of current expenditure in 2006.
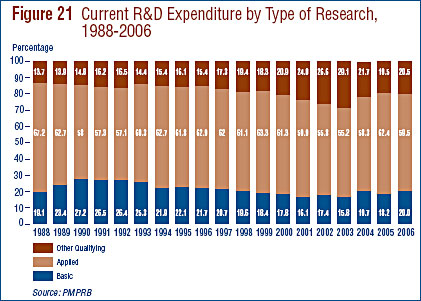
Current Expenditure by R&D Performer and by Source of Funds
Patentees report expenditure on research they conduct themselves (intramural) and research performed by other establishments, such as universities, hospitals and other manufacturers (extramural). Table 18 shows that slightly more than one-half (50.5%) of expenditure was intramural which declined from 52.6% intramural research in 2005. Research performed by other companies on behalf of patentees rose to 22.1% of current R&D expenditure in 2006, while the combined share of universities and hospitals was 16.2%.
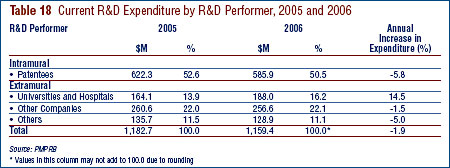
Patentees also report on the sources used to fund R&D expenditure. Table 19 shows that in 2006 patentees funded almost 87% of R&D expenditure with internal company funds.
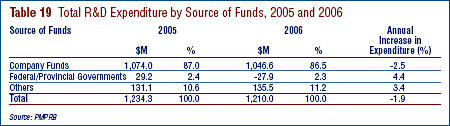
Current R&D Expenditure by Location
Table 20 breaks down current R&D expenditure by region. (See Table 23 in Annex 3, on page 61, for further detail on the division of current R&D expenditure among provinces and R&D performers.) As in previous years, expenditure was heavily concentrated in Ontario and Québec, these provinces accounting for 89.8% of total expenditure. R&D expenditure declined in all regions between 2005 and 2006.
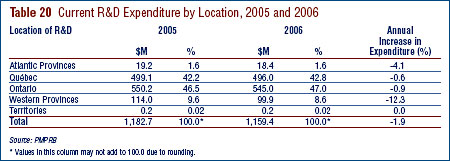
The Global Context
Figure 22 compares Canadian R&Dto- sales ratios to those of the seven comparator countries for the years 2000 and 2004.43 As noted above, Canada's ratio stood at 10.1% in 2000. Only Italy (6.2%) had a lower ratio in that year. Switzerland had the highest ratio at 102.5%, followed by Sweden at 44.4%. France, Germany and the U.S. were in the 16-18% range, while the U.K. was more than double (35.1%). A very similar pattern emerges in the ratios for 2004. Italy (6.6%) remained at the bottom of the range, with Canada second lowest at 8.3%. Ratios in all other comparator countries were again well above Canada's ratio, but showed declines in Switzerland and Sweden.
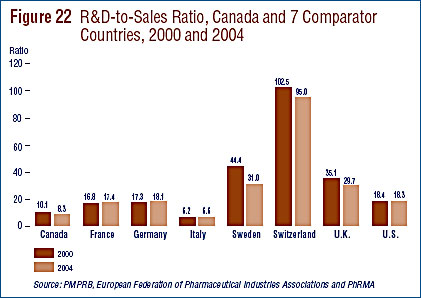
39 As per the Regulatory Impact Assessment Statement (RIAS) of the Patented Medicines Regulations, 1988, published in the Canada Gazette Part II, Vol. 122, No. 20 – SOR/DORS/88-474
40 The number of reporting companies varies from one year to the next.
41 Sales of drugs for both human and veterinary use are included for the purpose of this section of the report.
42 Current R&D expenditure consists of non-capital expenses directly related to research, including (a) wages and salaries, (b) direct material, (c) contractors and sub-contractors, (d) other direct costs such as factory overhead, (e) payments to designated institutions, (f) payments to granting councils and (g) payments to other organizations. These elements are described in more detail in the Patentees' Guide to Reporting – Form 3, available from the PMPRB Web site under Legislation, Regulations and Guidelines.
43 Sales in Figure 22 represent domestic sales and do not include exports.
The National Prescription Drug Utilization Information System (NPDUIS) provides critical analyses of price, utilization and cost trends so that Canada's health system has more comprehensive, accurate information on how prescription drugs are being used and on sources of cost increases. The Canadian Institute for Health Information (CIHI) and the PMPRB are partners in the NPDUIS. A steering committee, comprised of representatives of participating public drug plans and Health Canada, advises CIHI and the PMPRB on the development of the NPDUIS database and analytical studies. The NPDUIS initiative involves two major elements:
- development and implementation of a prescription claims level database capable of incorporating drug program data from participating publicly-funded drug plans; and
- production of analytical reports relying on information from this database.
CIHI is responsible for the first of these elements while, as requested by the Minister of Health under section 90 of the Patent Act, the PMPRB is principally responsible for the second.
The PMPRB has completed analyses of pharmaceutical trends from 1997-98 to 2004-05 based on aggregated DINlevel prescription drug data, provided by eight public drug plans in Canada.
Two projects have been recently developed to support informed decision-making:
- The Guidelines for Conducting Pharmaceutical Budget Impact Analyses for Submission to Public Drug Plans in Canada have been developed to provide guidance on the methodology and reporting standards to be used when submitting BIAs to the Common Drug Review, administered by Canadian Agency for Drugs and Technologies in Health (CADTH), or to federal, provincial or territorial drug plans that participate in the Common Drug Review (CDR). The Guidelines were published in May 2007.
- The New Drug Pipeline Monitor (NDPM) summarizes information on new drugs that are in the later phases of research and could have a significant impact in terms of therapeutic value. Future editions of the NDPM will track the clinical development of drugs selected for this web-based publication, highlight potential new drugs in the pipeline, and provide market analyses to inform decision-makers of potential cost impacts of the new drugs.
Studies conducted under the NPDUIS are available on the PMPRB Web site as is the list of ongoing projects.
In October 2005, the federal/provincial/ territorial (FPT) Ministers of Health announced the endorsement of the PMPRB to monitor and report on the prices of non-patented prescription drugs. In November 2005, the PMPRB received direction from the federal Minister of Health, on behalf of himself and his PT colleagues, to undertake this monitoring and reporting.
To-date, three reports have been released. Canadian and Foreign Price Trends, which examined domestic and international price and sales trends of non-patented prescription drugs, was released in July 2006; Trends in Canadian Sales and Market Structure was released in October 2006 and analyzed annual growth rates in sales, sources of growth, market shares, sales concentration, and international price comparisons by level of concentration. In June 2007, the third report on Non-Patented Prescription Drug Prices, Market for New Off-Patent Drugs was completed. This report examined brand name drug products that have gone off-patent between the years 2001 and 2003 and the degree and timing of entry of generic products. Reports are available on our Web site under Reporting; Non- Patented Prescription Drug Prices.
The fourth report, to be released this summer, will examine trends in prices of non-patented single-source prescription drugs sold in Canada and abroad. The analysis will cover issues such as recent developments in sales and prices, international price comparisons and an examination of factors determining market entry in Canada using a multivariate analysis.
Updates of each of these four reports will be published annually.
On December 31, 2005, proposed regulatory amendments were published in the Canada Gazette, Part 1. Following publication, numerous submissions were received from stakeholders which were carefully examined by the Board. As a result, the Board instructed Board Staff to prepare a revised package for submission for the Minister of Health's consideration in the fall.
As the revised regulatory package was in the final stages for submission to Treasury Board Cabinet Committee, Board Staff met with Rx&D, the Canadian Generic Pharmaceutical Association and BIOTECanada in February and March 2007 to discuss implementation of the proposed amendments. These discussions included the various revised forms patentees are required to file as part of their regulatory reporting requirements. In the context of these various meetings, the Industry raised specific concerns relating to a few proposed amendments, implementation of which could have increased the regulatory burden of patentees. As a result, the Board further considered these matters and instructed Board Staff to prepare a revised regulatory package for the Minister of Health's approval. This package will be forwarded to Treasury Board Cabinet Committee in the near future for publication in the Canada Gazette, Part II. The revised regulations will come into force on the day on which they are registered.
When the Regulations come into force, patentees will be informed of all changes to the filing requirements and will have access to updated forms which will be posted on our Web site.
In 2006, the Board launched a major initiative to engage stakeholders as part of its ongoing work to assess and consider potential modifications to its Excessive Price Guidelines (Guidelines) so that they remain effective in:
- facilitating Board Staff's review of patented drug prices; and
- promoting voluntary compliance on the part of patentees to ensure that prices of patented medicines sold in Canada are not excessive.
The release of the Discussion Guide on the Board's Excessive Price Guidelines (Guide) marked the first step in the initiative in May 2006. The Guide asked stakeholders to consider three issues: the categorization of new drugs; introductory price tests; and, the "any market" clause of the Patent Act (Act) in the price review process. The Board received 45 written submissions in response, reflecting the wide-ranging views of the individuals and groups affected by or interested in the Guidelines: patentees; patient and health care provider representatives; private and public insurance plans; members of the Human Drug Advisory Panel; academics; and consultants.
In November 2006, the Board met with close to 140 members of these stakeholder groups at sessions held in Edmonton, Montreal, Toronto, Halifax, and Ottawa. Participants deliberated on the topics of categories and "any market", as well as two new subjects: whether and when an introductory price should be "re-benched" (re-evaluated); and potential principles that could guide how the price factors in the Act are operationalized in the price review process. (The Guide and Summary Reports on each stakeholder meeting are available on the PMPRB's Web site at: www.pmprb-cepmb.gc.ca/consultations.)
The fundamental purpose of the Guidelines is to provide transparency and predictability in the price review process for all stakeholders. The Board recognizes that the pharmaceutical environment has evolved since the last major revision to the Guidelines in 1994, and that it is essential to ensure that the Guidelines remain relevant and appropriate in the current context. At the same time, it must be recognized that the Guidelines have been very effective in promoting voluntary compliance with non-excessive pricing. Currently, there are more than 1,100 patented drug products under the Board's jurisdiction. While a number of Notices of Hearing were issued and several investigations into apparent excessive prices were ongoing in 2006, the overall rate of compliance with the Guidelines for all patented drugs being sold in Canada is extremely high – at over 90%.
The Board is continuing its analysis in 2007. It has noted that the current Guidelines do not encompass all of the factors in the Act that the Board must consider in determining whether prices of patented medicines are excessive. For example, there is no guidance on the review of the second part of subsection 85(1)(c), "the prices at which …other medicines in the same therapeutic class have been sold in countries other than Canada."
Neither is there direction on subsection 85(2) – "Where, after taking into consideration the factors referred to in subsection (1), the Board is unable to determine whether the medicine is being or has been sold in any market in Canada at an excessive price, the Board may take into consideration the following factors: (a) the costs of making and marketing the medicine; and (b) such other factors as may be specified in any regulations made for the purposes of this subsection or as are, in the opinion of the Board, relevant in the circumstances." The current Guidelines are silent on guidance as to when a determination of whether prices are excessive based on subsection 85(1) may not be possible, on how the costs of making and marketing the medicine may be assessed, and on other factors that may be relevant.
The need to address these gaps has been added to the Board's overall workplan on the review of the current Guidelines. To further advance this work, and as announced in its April 2007 NEWSletter, the Board intends to hold bilateral consultations with groups representing sectors of the pharmaceutical industry, federal/ provincial/territorial governments and consumers. This process is expected to get underway during the summer of 2007.
Recognizing that this first major review of the Guidelines since 1994 may create a certain degree of uncertainty for patentees and other stakeholders regarding the future price review process, the Board is committed to ongoing open communication through its NEWSletter, its Web site and other means, as appropriate.
COMMUNICATIONS PROGRAM
Our Communications Program provides a framework for all key aspects of the PMPRB's strategy and practices. As an integral part of management, the Communications Program focuses on working in partnership with departments, stakeholders and the industry, when appropriate, to identify and convey pertinent information and to pursue the most appropriate course of action. It provides advice, develops strategies and helps inform and guide the decision-making process. Through sharing and networking, we seek to strengthen and enhance the effectiveness of our communications.
The Communications Program includes the development and maintenance of our communications policies, plans and activities. It is based on the concept of performancebased communications that are comprehensive, focused, clear, and consistent. In accordance with the Communications Policy of the Government of Canada, and related policies and guidelines, it is responsible for responding to public enquiries and is accountable for the management, direction and development of all communications activities, including media relations, and dissemination of information.
The Communications Program is responsive to the evolving requirements of the PMPRB's operating environment. Thus, it is the focus of continuous adjustment and improvement as we seek to find new and more effective ways of disseminating information about issues of interest to Canadians. We endeavour to ensure that the communications function brings a value-added dimension that advances the goals of the PMPRB.
In order to enhance our communications with stakeholders, we redesigned and are continuing to improve the PMPRB Web site to make it more informative and accessible while complying with government-wide standards.
Additional ongoing responsibilities of the Communications Program include: quarterly publication of the NEWSletter; coordinating the publication of studies and reports conducted under the NPDUIS; tracking Web site statistics in order to better understand the information needs of those visiting the PMPRB site; and, providing stakeholders direct access through the toll-free line and responding to general information requests.
The PMPRB is committed to pursue its engagement with stakeholders and ensure a two-way information exchange with them through a wide range of tools. Transparency and accessibility remain the central elements of the PMPRB's Communications Program.
PUBLICATIONS
We inform our stakeholders regularly through our publications. Our Annual Report and the NEWSletter are published at regular intervals throughout the year while other publications are released in response to program and corporate requirements.
| Publications |
January 2005 – May 2006 |
Release Date |
Annual Report |
June |
| NEWSletter |
Quarterly |
| Studies |
| |
NPDUIS
Pharmaceutical Trends Overview Report – 1997-1998 to 2003-2004 |
June 2006 |
| Budget Impact Analysis Guidelines |
May 2007 |
| New Drug Pipeline Monitoring (Web Report) |
June 2007 |
| Non-Patented Prescription Drug Prices Reports |
| |
Canadian and Foreign Price Trends |
June 2006 |
| Trends in Canadian Sales and Market Structure |
October 2006 |
| Markets for New Off-patent Drugs |
June 2007 |
| Hearings |
| |
Nicoderm – Hoechst Marion Roussel Canada Inc. |
1999 |
| Adderall XR, Shire BioChem Inc. |
2006 (ongoing) Dec 18 06: Decision on Shire's Motion on pre-patent |
| Airomir, 3M Canada Company |
2006 (see VCU, p. 16) |
| Concerta, Janssen-Ortho Inc. |
2006 (ongoing) |
| Copaxone, Teva Neuroscience G.P.-S.E.N.C. |
2006 (ongoing) |
| Dovobet, LEO Pharma Inc. |
2006 (ongoing) |
| Penlac Nail Lacquer, sanofi-aventis Canada Inc. |
2007 (ongoing) |
| Pentacel and Quadracel, sanofi pasteur Limited |
2007 (ongoing) |
| Risperdal Consta, Janssen-Ortho Inc. |
2006 (ongoing) |
| Strattera, Eli Lilly Canada Inc. |
2007 (ongoing) |
| Patented Medicines |
| |
Reported to the PMPRB in 2006 (including the review status for each drug) |
Monthly updates on Web site |
| Reports on New Patented Drugs: |
| |
Agenerase, GlaxoSmithKline Inc. |
February 2006 |
| Angiomax, Oryx Pharmaceuticals |
March 2006 |
| Avastin, Hoffmann-La Roche Limited, Canada |
January 2006 |
| Cancidas, Merck Frosst Canada |
May 2006 |
| Cipralex, Lundbeck Canada Inc. |
July 2006 |
| Elidel, Novaris Pharmaceuticals Canada Inc. |
June 2006 |
| Erbitux, Bristol-Myers Squibb Canada Inc |
July 2006 |
| Fuzeon, Hoffman-La Roche Limited, Canada |
October 2006 |
| Invanz, Merck Frosst Canada & Co. |
October 2006 |
| Keppra, Lundbeck Canada Inc. |
March 2006 |
| Ketek, sanofi-aventis Canada Inc. |
October 2006 |
| Levitra, Bayer Inc. |
March 2006 |
| Lyrica, Pfizer Canada Inc. |
January 2007 |
| Macugen, Pfizer Canada Inc. |
April 2007 |
| Remodulin, Northern Therapeutics Inc. |
June 2006 |
| Reyataz, Bristol-Myers Squibb Canada Inc. |
September 2006 |
| Sativex, Bayer Inc. |
November 2006 |
| Sensipar, Amgen Canada Inc. |
January 2006 |
| Solagé, Barrier Therapeutics Canada Inc. |
March 2006 |
| Somavert, Pfizer Canada Inc. |
November 2006 |
| Sutent, Pfizer Canada Inc. |
April 2007 |
| Tarceva, Hoffman-La Roche Limited, Canada |
July 2006 |
| TNKase, Hoffman-La Roche Limited, Canada |
October 2006 |
| Tramacet, Janssen-Ortho Inc. |
February 2006 |
| Vfend, Pfizer Canada Inc. |
October 2006 |
| Xolair, Novartis Pharmaceuticals Canada Inc. |
February 2006 |
| Zelnorm, Novartis Pharma Canada Inc. |
March 2006 |
| Speech Series |
| |
Examining the Flip Side of Drug Safety - Affordability
Presentation by Barbara Ouellet, at the Drug Safety Summit 2006, in Toronto |
February 2006 |
Pharmaceutical Pricing Environment and Regulation
Keynote Address by Barbara Ouellet, at the North American Pharma Summit, in Toronto |
March 2006 |
Summit on Pharmaceutical & Biotech Regulatory Compliance
Presentation by Barbara Ouellet |
March 2007 |
Standing Committee on Health on Main Estimates
Introductory Remarks by the Chairperson |
March 2007 |
| Voluntary Compliance Undertakings |
| |
NuvaRingTM , Organon Canada Ltd. |
June 2006 |
| Eloxatin, sanofi-aventis Canada Inc. |
July 2006 |
| Hextend, Hospira Healthcare Corporation |
July 2006 |
| Airomir, 3M Canada Company |
May 2007 |
This glossary is included for the convenience of the reader. For more detailed information and definitions please refer to the Patent Act, the Patented Medicines Regulations, the PMPRB Compendium of Guidelines, Policies and Procedures and the Food and Drug Regulations, or contact the PMPRB.
Active Ingredient:
Chemical or biological substance responsible for the claimed pharmacologic effect of a drug product. (Ingrédient actif)
Advance Ruling Certificate (ARC):
A non-binding advance ruling certificate may be issued pursuant to subsection 98(4) of the Patent Act at the request of a patentee when the Board is satisfied that the price or proposed price of the medicine would not exceed the maximum non-excessive price under the Board's Guidelines.
ATC:
Anatomical Therapeutic Chemical (ATC) classification system, developed and maintained by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology, divides drugs into different groups according to their site of action and therapeutic and chemical characteristics. This system is used by the PMPRB as a guide for selecting comparable medicines for purposes of price review.
Dedication of Patent:
A practice whereby a patentee notifies the Commissioner of Patents that it has surrendered its rights and entitlements flowing from the patent for the benefit of the public to use and enjoy. NB: As of January 30, 1995, the Board does not recognize dedication of patent as a means to remove the medicine from its jurisdiction. (See PMPRB Bulletin 17, page 3.)
Drug Identification Number (DIN):
A registration number (drug identification number) that the Health Products and Food Branch of Health Canada assigns to each prescription and non-prescription drug product marketed under the Food and Drug Regulations. The DIN is assigned using information in the following areas: manufacturer of the product; active ingredient(s); strength of active ingredient(s); pharmaceutical dosage form; brand/trade name; and route of administration.
Drug Product:
A particular presentation of a medicine characterized by its pharmaceutical dosage form and the strength of the active ingredient(s).
Drug Product, Existing:
An existing drug product is a DIN for which a benchmark price has been established in accordance with the Board's Guidelines. (See Chapter 1, subsection 3.3 of the Compendium of Guidelines, Policies and Procedures.)
Drug Product, New:
A new drug product is one for which the introductory price is under review. Patented drug products are considered new in the year during which they are first introduced on the market in Canada or the year they receive their first patent(s) if previously marketed. For price review purposes, new drug products for a given year are those introduced between December 1, of the previous year and November 30, of the reporting year. Because of the filing requirements under the Patented Medicines Regulations and the manner of calculating benchmark prices, drug products introduced in December are considered to have been introduced in the following year. (See Chapter 1, subsection 3.2 of the Compendium of Guidelines, Policies and Procedures.)
Emergency Drug Release (EDR) Program:
See Special Access Programme.
Generic Product:
A drug product with the same active ingredient, strength and dosage form of a brand name drug product.
Investigational New Drug (IND):
A drug that has been authorized for clinical evaluation (i.e. testing on humans) by Health Canada but that is not yet approved for sale for the indication under study.
License, Compulsory:
Referred to in subsection 79(1) of the Patent Act, means a license granted by the Commissioner of Patents, before December 20, 1991, in accordance with subsection 39(4) of the Patent Act, R.S., 1985, c. P-4 that has been continued pursuant to subsection 11(1) of the Patent Act Amendment Act, 1992 which permits the licensee to import, make, use or sell a patented invention pertaining to a medicine. Royalties payable are determined by the Commissioner of Patents who sets the terms of licenses pursuant to subsection 39(5) of the Patent Act.
License, Voluntary:
A contractual agreement between a patent holder and a licensee under which the licensee is entitled to enjoy the benefit of the patent or to exercise any rights in relation to the patent for some consideration (i.e., royalties in the form of a share of the licensee's sales.)
Medicine:
Any substance or mixture of substances made by any means, whether produced biologically, chemically, or otherwise, that is applied or administered in vivo in humans or in animals to aid in the diagnosis, treatment, mitigation or prevention of disease, symptoms, disorders, abnormal physical states, or modifying organic functions in humans and or animals, however administered. For greater certainty, this definition includes vaccines, topical preparations, anaesthetics and diagnostic products used in vivo, regardless of delivery mechanism (e.g. transdermal, capsule form, injectable, inhaler, etc.). This definition excludes medical devices, in vitro diagnostic products and disinfectants that are not used in vivo. (See Compendium of Guidelines, Policies and Procedures, Introduction, subsection 1.5.)'
Notice of Compliance (NOC):
A notice in respect of a medicine issued by the Health Products and Food Branch of Health Canada under section C.08.004 of the Food and Drugs Regulations. The issuance of a NOC indicates that a drug product meets the required Health Canada standards for use in humans or animals and that the product is approved for sale in Canada.
Patent:
An instrument issued by the Commissioner of Patents in the form of letters patent for an invention that provides its holder with a monopoly limited in time, for the claims made within the patent. A patent gives its holder and its legal representatives, the exclusive right of making, constructing and using the invention and selling it to others to be used.
Patented Medicine Price Index (PMPI):
The PMPI has been developed by the PMPRB as a measure of average year-over-year change in the transaction prices of patented drug products sold in Canada, based on the price and sales information reported by patentees.
Patentee:
As defined by subsection 79(1) of the Patent Act, "the person for the time being entitled to the benefit of the patent for that invention and includes, where any other person is entitled to exercise any rights in relation to that patent other than under a license continued by subsection 11(1) of the Patent Act Amendment Act, 1992, that other person in respect of those rights;"
Pending Patent:
An application for a patent that has not yet been issued.
NB: In cases where a medicine is sold before a patent is issued, it is the Board's policy once the patent is issued, to review the price of the medicine as of the date on which the patent application was laid open for public inspection. (See PMPRB Bulletin 15, page 7.)
Research and Development (R&D):
Basic or applied research for the purpose of creating new, or improving existing, materials, devices, products or processes (e.g. manufacturing processes).
Research and Development - Applied Research:
Work that advances scientific knowledge with a specific practical application in view such as creating new or improved products or processes through manufacturing processes or through preclinical or clinical studies.
Research and Development - Basic Research:
Work that advances scientific knowledge without a specific application in view.
Research and Development - Clinical Research:
The assessment of the effect of a new medicine on humans. It typically consists of three successive phases, beginning with limited testing for safety in healthy humans then proceeding to further safety and efficacy studies in patients suffering from the target disease.
Research and Development - Preclinical Research:
Tests on animals to evaluate the pharmacological and toxicological effects of medicines.
Research and Development - Other Qualifying:
Includes eligible research and development expenditures that cannot be classified into any of the preceding categories of "type of research and development". It includes drug regulation submissions, bioavailability studies and Phase IV clinical trials.
Research and Development Expenditures:
For the purposes of the Patented Medicines Regulations, 1994, in particular sections 5 and 6, research and development includes activities for which expenditures would have qualified for the investment tax credit for scientific research and experimental development under the Income Tax Act as it read on December 1, 1987.
Research and Development Expenditures - Current:
Consist of the following non-capital expenses that are directly related to research work: (a) wages and salaries, (b) direct material, (c) contractors and subcontractors, (d) other direct costs such as factory overhead, (e) payments to designated institutions, (f) payments to granting councils and (g) payments to other organizations. These elements are described in greater detail in the Patentees´ Guide to Reporting - Form 3 available from the PMPRB Web site under "Legislation, Regulations and Guidelines."
Special Access Program (SAP):
A program operated by Health Canada to give practitioners access to drugs that are not approved or otherwise available for sale in Canada. (Formerly the EDR Program.) Voluntary Compliance
Undertaking (VCU):
A written undertaking by a patentee to adjust its price to conform to the PMPRB's Excessive Price Guidelines (see Chapter 1 of the Compendium of Guidelines, Policies and Procedures). Pursuant to the Board's Compliance and Enforcement Policy (see Chapter 2, section 7) the Chairperson may approve a VCU in lieu of issuing a Notice of Hearing if it is consistent with the Patent Act and the policies of the Board and in the public interest. Under the Board's Compliance and Enforcement Policy, a VCU can also be submitted following the issuance of a Notice of Hearing. A VCU submitted at this point must be approved by the Board. The Board reports publicly on all VCUs approved by the Chairperson or the Board.
A price is considered to be within the Guidelines unless it meets the criteria for commencing an investigation. The criteria represent the standards the Board applies in order to allocate its resources to investigations as efficiently as possible. Their existence should not be construed as indicating that the Board accepts any deviation from the Guidelines. The Board is satisfied that its criteria assure all significant cases of pricing outside the Guidelines will be subject to investigation. In most instances where a price exceeds the maximum allowable price by an amount too small to trigger an investigation in one year, it is offset by a price below that which is permitted by the Guidelines the following year. The Board expects the prices of all patented medicines to be within the Guidelines and evidence of persistent pricing outside the Guidelines, even by a small amount, may be used as a criterion for commencing an investigation.
Board Staff will commence an investigation into the price of a patented drug product when any of the following criteria are met:
New Drug Products
- The introductory price is 5% or more above the maximum nonexcessive price;
- Excess revenues in the introductory period are $25,000 or more; or
- Complaints with significant evidence.
Existing Drug Products
- A price is 5% or more above the maximum non-excessive price and there are cumulative excess revenues of $25,000 or more over the life of the patent after January 1, 1992;
- Cumulative excess revenues are $50,000 or more over the life of the patent after January 1, 1992; or
- Complaints with significant evidence.
For more information on the Criteria for Commencing an Investigation, please consult Schedule 5 of the Compendium of Guidelines, Policies and Procedures available on the PMPRB Web site under Legislation, Regulations, Guidelines.
| BRAND NAME |
COMPANY |
DIN |
NAS1/FPG2 |
ATC3 |
STATUS |
CATEGORY |
| Actonel Plus Calcium 35 mg/500 mg/tablet |
Proctor & Gamble Pharmaceuticals Canada Inc. |
02279657 |
|
M |
Within Guidelines |
3 |
| Advate 250 unit/vial |
Baxter Corporation |
02284138 |
|
B |
Within Guidelines |
1 |
| Advate 500 unit/vial |
Baxter Corporation |
02284146 |
|
B |
Within Guidelines |
1 |
| Advate 1000 unit/vial |
Baxter Corporation |
02284154 |
|
B |
Within Guidelines |
1 |
| Advate 1500 unit/vial |
Baxter Corporation |
02284162 |
|
B |
Within Guidelines |
1 |
| Advicor 500/20 520 mg/tablet |
Oryx Pharmaceuticals Inc. |
02270439 |
|
C |
Within Guidelines |
3 |
| Advicor 1000/20 1020 mg/tablet |
Oryx Pharmaceuticals Inc. |
02270447 |
|
C |
Within Guidelines |
3 |
| Altace 15 mg/capsule |
sanofi-aventis Canada Inc. |
02281112 |
|
C |
Within Guidelines |
1 |
| Altace HCT 15 mg/tablet |
sanofi-aventis Canada Inc. |
02283131 |
|
C |
Within Guidelines |
3 |
| Altace HCT 17.5 mg/tablet |
sanofi-aventis Canada Inc. |
02283158 |
|
C |
Within Guidelines |
3 |
| Altace HCT 22.5 mg/tablet |
sanofi-aventis Canada Inc. |
02283166 |
|
C |
Within Guidelines |
3 |
| Altace HCT 30 mg/tablet |
sanofi-aventis Canada Inc. |
02283174 |
|
C |
Within Guidelines |
3 |
| Altace HCT 35 mg/tablet |
sanofi-aventis Canada Inc. |
02283182 |
|
C |
Within Guidelines |
3 |
| Alvesco 100 mcg/dose |
Altana Pharma Inc. |
02285606 |
NAS |
R |
Under Review |
|
| Alvesco 200 mcg/dose |
Altana Pharma Inc. |
02285614 |
NAS |
R |
Under Review |
|
| Andriol 40 mg/capsule |
Organon Canada Ltd. (AKZO) |
00782327 |
FPG |
G |
Under Review |
|
| Aptivus 250 mg/capsule |
Boehringer Ingelheim (Canada) Ltd. |
02273322 |
NAS/FPG |
J |
Within Guidelines |
3 |
| Arava 10 mg/tablet |
sanofi-aventis Canada Inc. |
02241888 |
NAS/FPG |
L |
Under Review |
|
| Arava 20 mg/tablet |
sanofi-aventis Canada Inc. |
02241889 |
NAS/FPG |
L |
Under Review |
|
| Aricept RDT 5 mg/tablet |
Pfizer Canada Inc. |
02269457 |
|
N |
Within Guidelines |
1 |
| Aricept RDT 10 mg/tablet |
Pfizer Canada Inc. |
02269465 |
|
N |
Within Guidelines |
1 |
| Avalide 300/25 325 mg/tablet |
Bristol-Myers Squibb Canada Co. |
02280213 |
|
C |
Within Guidelines |
1 |
| Avandaryl 4/1 5 mg/tablet |
GlaxoSmithKline Inc. |
02258781 |
|
A |
Under Review |
|
| Avandaryl 4/2 6 mg/tablet |
GlaxoSmithKline Inc. |
02258803 |
|
A |
Under Review |
|
| Avandaryl 4/4 8 mg/tablet |
GlaxoSmithKline Inc. |
02258811 |
|
A |
Under Review |
|
| Azilect 0.5 mg/tablet |
Teva Neuroscience |
02284642 |
NAS |
N |
Under Investigation |
|
| Azilect 1 mg/tablet |
Teva Neuroscience |
02284650 |
NAS |
N |
Under Investigation |
|
| Baraclude 0.05 mg/ml |
Bristol-Myers Squibb Canada Co. |
02282232 |
NAS |
J |
Within Guidelines |
3 |
| Baraclude 0.5 mg/tablet |
Bristol-Myers Squibb Canada Co. |
02282224 |
NAS |
J |
Within Guidelines |
3 |
| Caduet 10/10 20 mg/tablet |
Pfizer Canada Inc. |
02273284 |
|
C |
Within Guidelines |
3 |
| Caduet 10/20 30 mg/tablet |
Pfizer Canada Inc. |
02273292 |
|
C |
Within Guidelines |
3 |
| Caduet 10/40 50 mg/tablet |
Pfizer Canada Inc. |
02273306 |
|
C |
Within Guidelines |
3 |
| Caduet 10/80 90 mg/tablet |
Pfizer Canada Inc. |
02273314 |
|
C |
Within Guidelines |
3 |
| Caduet 5/10 15 mg/tablet |
Pfizer Canada Inc. |
02273233 |
|
C |
Within Guidelines |
3 |
| Caduet 5/20 25 mg/tablet |
Pfizer Canada Inc. |
02273241 |
|
C |
Within Guidelines |
3 |
| Caduet 5/40 45 mg/tablet |
Pfizer Canada Inc. |
02273268 |
|
C |
Within Guidelines |
3 |
| Caduet 5/80 85 mg/tablet |
Pfizer Canada Inc. |
02273276 |
|
C |
Within Guidelines |
3 |
| Clobex Lotion 0.5 mg/ml |
Galderma Canada Inc. |
02256398 |
|
D |
Within Guidelines |
1 |
| Cubicin 500 mg/vial |
Oryx Pharmaceuticals Inc. |
|
NAS |
J |
Under Review |
|
| DDAVP Melt 60 mcg/tablet |
Ferring Pharmaceuticals Inc. |
02284995 |
|
H |
Under Investigation |
|
| DDAVP Melt 120 mcg/tablet |
Ferring Pharmaceuticals Inc. |
02285002 |
|
H |
Under Investigation |
|
| Denavir 10 mg/gm |
Barrier Therapeutics Canada Inc. |
02238848 |
NAS |
D |
Under Review |
|
| Diastat 5 mg/ml |
Shire BioChem Inc. |
02238162 |
FPG |
N |
Under Review |
|
| Duo Trav .04/5 5.04 mg/ml |
Alcon Canada Inc. |
02278251 |
|
S |
Within Guidelines |
3 |
| Enablex 7.5 mg/tablet |
Novartis Pharma Canada Ltd. |
02273217 |
NAS |
G |
Within Guidelines |
3 |
| Enablex 15 mg/tablet |
Novartis Pharma Canada Ltd. |
02273225 |
NAS |
G |
Within Guidelines |
3 |
| Enbrel 50 mg/syringe |
Amgen Canada Inc. |
02274728 |
|
L |
Under Investigation |
|
| Exjade 125 mg/tablet |
Novartis Pharma Canada Ltd. |
02287420 |
NAS |
V |
Under Review |
|
| Exjade 250 mg/tablet |
Novartis Pharma Canada Ltd. |
02287439 |
NAS |
V |
Under Review |
|
| Exjade 500 mg/tablet |
Novartis Pharma Canada Ltd. |
02287447 |
NAS |
V |
Under Review |
|
The Board's Guidelines establish three categories of new patented drug products for purposes of conducting introductory price reviews.
- Category 1 – a new DIN of an existing or comparable dosage form of an existing medicine, usually a new strength of an existing drug (line extension)
- Category 2 – the first drug to treat effectively a particular illness or which provides a substantial improvement over existing drug products, often referred to as "breakthrough" or substantial improvement.
- Category 3 – a new drug or new dosage form of an existing medicine that provides moderate, little or no improvement over existing medicines.
For complete definitions of the categories, refer to the Compendium of Guidelines, Policies and Procedures, Chapter 3, section 3.
- NAS: New Active Substance
- FPG: First Patent Grant
- ATC: Anatomical Therapeutic Chemical Classification System
| BRAND NAME |
COMPANY |
DIN |
NAS1/FPG2 |
ATC3 |
STATUS |
CATEGORY |
| Faslodex 250 mg/syringe |
AstraZeneca Canada Inc. |
02248624 |
NAS |
L |
Under Investigation |
|
| Flomax CR 0.4 mg/tablet |
Boehringer Ingelheim (Canada) Ltd. |
02270102 |
|
G |
Within Guidelines |
1 |
| Fosavance 70 mg/tablet |
Merck Frosst Canada Ltd. |
02276429 |
|
M |
Within Guidelines |
3 |
| Fuzeon 108 mg/vial |
Hoffmann-La Roche Ltd. Canada |
02247725 |
NAS/ FPG |
J |
Within Guidelines |
2 |
| Gardasil 0.5 ml/vial |
Merck Frosst Canada Ltd. |
02283190 |
NAS |
J |
Within Guidelines |
1 |
| Glumetza 500 mg/tablet |
Biovail Pharmaceuticals Canada |
02268493 |
FPG |
A |
Under Investigation |
|
| Hepsera 10 mg/tablet |
Gilead Sciences Inc. |
02247823 |
NAS |
J |
Within Guidelines |
3 |
| Idamycin PFS 1 mg/ml |
Pfizer Canada Inc. |
02282496 |
|
L |
Within Guidelines |
1 |
| Invirase 500 mg/tablet |
Hoffmann-La Roche Ltd. Canada |
02279320 |
|
J |
Within Guidelines |
1 |
| Kaletra 200/50 250 mg/tablet |
Abbott Laboratories Ltd. |
02285533 |
|
J |
Within Guidelines |
1 |
| Kogenate FS Bioset 250 |
Bayer Inc. |
02254476 |
|
B |
Within Guidelines |
1 |
| Lantus 100 unit/ml |
sanofi-aventis Canada Inc. |
02251930 |
|
A |
Under Investigation |
|
| Levemir Penfill 100 unit/ml |
Novo Nordisk Canada Inc. |
02271842 |
NAS |
A |
Under Investigation |
|
| Macugen 0.3 mg/vial |
Pfizer Canada Inc. |
02267225 |
NAS/ FPG |
S |
Within Guidelines |
2 |
| Myfortic 180 mg/tablet |
Novartis Pharma Canada Ltd. |
02264560 |
FPG |
L |
Within Guidelines |
1 |
| Myfortic 360 mg/tablet |
Novartis Pharma Canada Ltd. |
02264579 |
FPG |
L |
Within Guidelines |
1 |
| Niaspan 500 mg/tablet |
Oryx Pharmaceuticals Inc. |
02262347 |
FPG |
C |
Within Guidelines |
3 |
| Niaspan 750 mg/tablet |
Oryx Pharmaceuticals Inc. |
02262355 |
FPG |
C |
Within Guidelines |
3 |
| Niaspan 1000 mg/tablet |
Oryx Pharmaceuticals Inc. |
02262339 |
FPG |
C |
Within Guidelines |
3 |
| Nutrineal PD4 11 mg/ml |
Baxter Corporation |
02230810 |
NAS/ FPG |
B |
Under Review |
|
| Pantoloc M 40 mg/tablet |
Altana Pharma Inc. |
02267233 |
|
A |
Within Guidelines |
1 |
| Prevacid Fastab 30 mg/tablet |
Abbott Laboratories Ltd. |
02249472 |
|
A |
Under Investigation |
|
| Prezista 300 mg/tablet |
Janssen-Ortho Inc. |
02284057 |
NAS |
J |
Within Guidelines |
3 |
| Revatio 20 mg/tablet |
Pfizer Canada Inc. |
02279401 |
|
G |
Within Guidelines |
1 |
| Risperdal M-Tab 3 mg/tablet |
Janssen-Ortho Inc. |
02268086 |
|
N |
Within Guidelines |
1 |
| Risperdal M-Tab 4 mg/tablet |
Janssen-Ortho Inc. |
02268094 |
|
N |
Within Guidelines |
1 |
| Rotateq 2 ml/dose |
Merck Frosst Canada Ltd. |
02284413 |
NAS |
J |
Within Guidelines |
2 |
| Sativex 27/25 52 mg/ml |
Bayer Inc. |
02266121 |
NAS/ FPG |
N |
Within Guidelines |
3 |
| Somavert 10 mg/vial |
Pfizer Canada Inc. |
02272199 |
NAS |
H |
Within Guidelines |
3 |
| Somavert 15 mg/vial |
Pfizer Canada Inc. |
02272202 |
NAS |
H |
Within Guidelines |
3 |
| Somavert 20 mg/vial |
Pfizer Canada Inc. |
02272210 |
NAS |
H |
Within Guidelines |
3 |
| Sutent 12.5 mg/capsule |
Pfizer Canada Inc. |
02280795 |
NAS |
L |
Within Guidelines |
3 |
| Sutent 25 mg/capsule |
Pfizer Canada Inc. |
02280809 |
NAS |
L |
Within Guidelines |
3 |
| Sutent 50 mg/capsule |
Pfizer Canada Inc. |
02280817 |
NAS |
L |
Within Guidelines |
3 |
| Thalomid 50 mg/capsule |
Celegene Corporation |
|
NAS/ FPG |
L |
Under Review |
|
| Trelstar 3.75 mg/vial |
Paladin Labs Inc. |
02240000 |
NAS |
L |
Within Guidelines |
3 |
| Trelstar LA 11.25 mg/vial |
Paladin Labs Inc. |
02243856 |
NAS |
L |
Within Guidelines |
3 |
| Trileptal 150 mg/tablet |
Novartis Pharma Canada Ltd. |
02242067 |
NAS/ FPG |
N |
Under Review |
|
| Trileptal 300 mg/tablet |
Novartis Pharma Canada Ltd. |
02242068 |
NAS/ FPG |
N |
Under Review |
|
| Trileptal 600 mg/tablet |
Novartis Pharma Canada Ltd. |
02242069 |
NAS/ FPG |
N |
Under Review |
|
| Trusopt PF 20 mg/ml |
Merck Frosst Canada Ltd. |
02269090 |
|
S |
Within Guidelines |
1 |
| Truvada 200/300 500 mg/tablet |
Gilead Sciences Inc. |
0227490 |
|
J |
Within Guidelines |
3 |
| Tygacil 50 mg/vial |
Wyeth Pharmaceuticals |
02285401 |
NAS |
J |
Under Review |
|
| Tysabri 20 mg/ml |
Biogen Idec Canada Inc. |
02286386 |
NAS |
L |
Under Investigation |
|
| Vantas 50 mg/imp |
Paladin Labs Inc. |
02278383 |
NAS |
H |
Within Guidelines |
3 |
| Vesicare 5 mg/tablet |
Astellas Pharma Canada Inc. |
02277263 |
NAS |
G |
Within Guidelines |
3 |
| Vesicare 10 mg/tablet |
Astellas Pharma Canada Inc. |
02277271 |
NAS |
G |
Within Guidelines |
3 |
| Wellbutrin XL 150 mg/tablet |
Biovail Pharmaceuticals Canada |
02275090 |
|
N |
Within Guidelines |
1 |
| Wellbutrin XL 300 mg/tablet |
Biovail Pharmaceuticals Canada |
02275104 |
|
N |
Within Guidelines |
1 |
The Board's Guidelines establish three categories of new patented drug products for purposes of conducting introductory price reviews.
- Category 1 – a new DIN of an existing or comparable dosage form of an existing medicine, usually a new strength of an existing drug (line extension)
- Category 2 – the first drug to treat effectively a particular illness or which provides a substantial improvement over existing drug products, often referred to as "breakthrough" or substantial improvement.
- Category 3 – a new drug or new dosage form of an existing medicine that provides moderate, little or no improvement over existing medicines.
For complete definitions of the categories, refer to the Compendium of Guidelines, Policies and Procedures, Chapter 3, section 3.
- NAS: New Active Substance
- FPG: First Patent Grant
- ATC: Anatomical Therapeutic Chemical Classification System

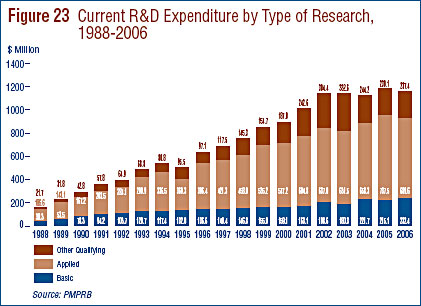

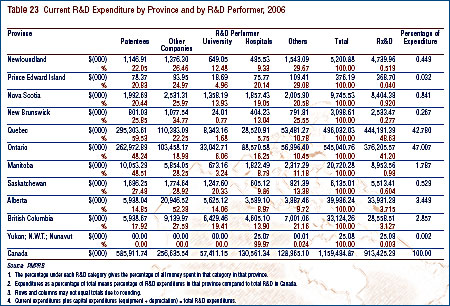
Back To Table of Contents