2004 Annual Report
Readers will note that in this Annual Report the Reporting Information on Key Pharmaceutical Trends section has been re-organized and new tables and figures have been added. It is hoped that the more in-depth analysis of the key indices will assist the readers in better understanding the current context. These changes are very much in line with the reporting mandate of the PMPRB to contribute to informed decisions and policy making by reporting, among others, on pharmaceutical trends.

Download PDF
Table Of Contents
- Letter to the Minister of Health
- Highlights for 2004
- Chairperson's Message
- About the Patented Medicine Prices Review Board: Mandate and Jurisdiction
- Regulating Prices of Patented Medicines
- Advance Ruling Certificate
- Voluntary Compliance Undertakings
- Quasi-judicial Activities
- Reporting Information on Key Pharmaceutical Trends
- Trends in Sales
- Price Trends
- Comparison of Canadian Prices to Foreign Prices
- Utilization of Patented Drugs
- Manufacturing Trends in Canada
- The Global Context
- National Prescription Drug Utilization Information System
- Analysis of Research-and-Development (R&D) Expenditures
- Policy and Research Initiatives
- Governance
- Members' Biographies
- Budget
- Publications
- Glossary
- Acronyms
- Annex 1
- Annex 2
- Annex 3
- Patented Drug Products for Human Use and Canadian Patentees
- Footnotes
May 31, 2005
The Honourable Ujjal Dosanjh, P.C., M.P.
Minister of Health
House of Commons
Ottawa, Ontario
K1A 0A6
Dear Minister:
I have the honour to present to you, in accordance with sections 89 and 100 of the Patent Act, the Annual Report of the Patented Medicine Prices Review Board for the year ended December 31, 2004.
Yours very truly,
Réal Sureau
Vice-Chairperson
Sales
- Total sales of all drugs for human use by manufacturers in Canada increased 5.3% from 2003 to $15.9 billion. The growth rate in 2004 is the lowest seen since 1997.
- Sales of patented drugs increased by 7.9% to $10.9 billion in 2004. The growth rate is the lowest seen since 1996.
- Patented drugs account for 68.6% of total drug sales, slightly higher than last year.
Compliance
- In total, there were 94 new patented drug products introduced in 2004, including 25 new active substances. As of March 31, 2005, 90 new patented drug products had been reviewed. Of those, 68 were considered to be within the Guidelines; twenty two are subject to ongoing investigations.
Enforcement
- The Board issued three Notices of Hearing and approved eight Voluntary Compliance Undertakings.
Pharmaceutical Trends
- The manufacturers' prices of patented drugs, as measured by the Patented Medicine Price Index (PMPI) fell by 0.2% in 2004. Analysis of prices by therapeutic class demonstrates considerable variability in price changes.
- In 2004, the ratio of Canadian prices to the international median for comparator countries was again below parity, with Canadian patented drug prices being on average about 91% of the corresponding median international price. Prices of patented drugs in Canada were on average somewhat less than prices in Sweden, Germany, the U.K. and Switzerland, but greater than prices in France and Italy. As in previous years, U.S. prices appear to be substantially higher than prices in both Europe and Canada.
Research and Development
- Patentees reported total R&D expenditures of $1.17 billion in 2004, a decrease of 2% over the $1.19 billion in the previous year. Rx&D members reported R&D expenditure of $1,008.3 million in 2004, accounting for 86.2% of all reported expenditures.
- The R&D-to-sales ratio for all patentees declined to 8.3% in 2004 from 8.8% in 2003 as did the R&D-to-sales ratio for members of Rx&D to 8.5% from 9.1% the previous year. R&D-to-sales ratios for all patentees and for Rx&D members have declined in recent years, after rising from 1988 to the mid-1990s. Whether taken over all patentees or restricted to Rx&D members, R&D-to-sales ratios in 2004 were the lowest seen since 1989.
- Patentees reported spending $221.7 million on basic research, representing 19.7% of current R&D expenditure. Spending on basic research increased by 23% in 2004 relative to the previous year.
It is my distinct pleasure to sign the Chairperson's Message of the Patented Medicine Prices Review Board's Annual Report for 2004.
As a reflection of Canada's dynamic health care environment, the PMPRB has experienced significant changes over the past year. Perhaps the most important of these changes has been our continuing evolution as an organization. After a decade of leadership and service as Chairperson of the PMPRB, Dr. Robert G. Elgie has completed his mandate. One of Dr. Elgie's strongest influences was as someone who challenged those around him to continue to study and debate the issues and policies that shape the work of the PMPRB. He achieved a great deal during his term. The proof of that is that the PMPRB has continually met its objective of protecting Canadian consumers from excessive drug prices.
As Vice-Chairperson, I am honoured to assume the responsibilities of the Chairperson until such time as a new Chair is appointed. In this capacity, I will be guided by Dr. Elgie's example and will continue to pursue the values and mission of the PMPRB.
Pharmaceuticals remain front and centre in public policy discussions. Canada's health care system, of which the PMPRB is a key part, has served to ensure that consumers are protected from excessive prices for patented medicines. In 1987, Canadian prices for patented drugs were second highest in the world, 23% above the median of foreign prices and higher than the six European countries used for comparison purposes. After the creation of the PMPRB and the introduction of its Guidelines, that ratio declined but Canadian prices were still approximately 10% above the median in the early 1990s. Concerned that it had not achieved its objective, the Board amended its Guidelines effective in 1994. Since then, Canadian prices have consistently been between 5% to 12% below the median of foreign prices.
In 2004, the PMPRB became aware of reports in the media of price increases and began to receive questions from public drug plans about price announcements they had received. In all, we received information that a number of manufacturers of patented medicines had made public announcements of price increases. If such increases were to come about, they could represent a change in the trends in pricing in Canada over the past decade. Consequently, we undertook a public consultation on price increases and issued a discussion paper. We are currently reviewing stakeholders' submissions following which the Board will determine next steps.
Over the last decade, the patented drug sector has grown significantly - their share of total sales in Canada having increased from 45% in 1996 to over 68% in 2004. By 2004, the growth in total sales of all drugs reached close to $16 billion. These increases were reflected in expenditures by governments and by consumers through their private insurance coverage and as out-of-pocket costs.
In its latest report, the Canadian Institute for Health Information (CIHI) estimated that total expenditures by Canadians on medicines reached $22 billion in 2004 and that drugs now represent nearly 17% of total health care spending in Canada. Public programs have sought a greater understanding of the reasons for such growth and whether it is appropriate. They have introduced new approaches to contain costs and they have sought out new approaches to collaboration.
Increasingly, the PMPRB has been asked to do more to focus on the broader questions relating to drug costs. Our previous studies have shown that the major factors driving up drug costs have been the impact of the introduction of new drugs and increased utilization of drugs in the health care system.
In the context of its role in the National Prescription Drug Utilization Information System (NPDUIS), the PMPRB has undertaken a number of initiatives. The NPDUIS provides critical analyses of price, utilization and cost trends so that our health system has more comprehensive, accurate information on how prescription drugs are being used and on sources of cost increases. Currently, we are involved in a number of projects that will supply the participating jurisdictions with such information.
As demonstrated by the NPDUIS, collaboration among governments is an important element in addressing health care issues that affect all Canadians. In September, the First Ministers agreed to build on this collaboration by developing and implementing a National Pharmaceuticals Strategy as part of their comprehensive agreement on health care. They declared that: "Affordable access to drugs is fundamental to equitable health outcomes for all our citizens." A Ministerial Task Force is focusing on a number of key areas relating to, among others, catastrophic drug coverage; introduction of a national drug formulary; improving access to breakthrough drugs; and accelerating access to non-patented drugs and achieving international parity on prices of non patented drugs.
Not only is greater collaboration taking place among the various levels of government in Canada but also among all participants in the health care system to improve pharmaceuticals management in the coming years.
The PMPRB is proud of the contribution it has made to ensure Canadians do not pay excessive prices for patented medicines. We are dedicated to continuing to work with our partners and stakeholders in the interests of all Canadians.
In closing, I would like to take this opportunity to welcome Dr. Brien Benoit who was appointed to the Board on May 19. I welcome back Dr. Anthony Boardman who was appointed to the Board for a second term. Also, I welcome Barbara Ouellet, who joined the PMPRB in January as Executive Director.
Also, I wish to thank Dr. Ingrid Sketris for her invaluable participation as Board Member. She completed her mandate with the Board in May 2004. I would be remiss if I did not thank the talented and dedicated staff of the PMPRB for their contribution to this Program. In particular, I want to thank Wayne Critchley, who has served the PMPRB as Executive Director for 15 years. He made an outstanding contribution to this organization for which we are grateful. I offer my best wishes of success to our former and new colleagues in their endeavours.
Réal Sureau
Vice-Chairperson
The Patented Medicine Prices Review Board is an independent quasi-judicial body established by Parliament in 1987 under the Patent Act (Act). The Minister of Health is responsible for the pharmaceutical provisions of the Act as set out in sections 79 to 103.
Although the PMPRB is part of the Health Portfolio, it carries out its mandate at arms-length from the Minister of Health.[1] It also operates independently of other bodies such as Health Canada, which approves drugs for safety and efficacy, and public drug plans, which approve the listing of drugs on their respective formularies for reimbursement purposes.
Mandate
The PMPRB has a dual role:
- Regulatory - To protect consumers and contribute to Canadian health care by ensuring that prices charged by manufacturers for patented medicines are not excessive;
- Reporting - To contribute to informed decisions and policy making, by reporting on pharmaceutical trends and on the R&D spending by pharmaceutical patentees.
Jurisdiction
Regulatory - The PMPRB is responsible for regulating the prices that patentees charge, the "factory-gate" price, for prescription and non-prescription patented drugs sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, to ensure that they are not excessive. The PMPRB regulates the price of each patented drug product, including each strength of each dosage form of each patented medicine sold in Canada. This is normally the level at which Health Canada assigns a Drug Identification Number (DIN).
In Canada, Health Canada assesses new medicines to ensure that they conform with the Food and Drugs Act and Regulations. Formal authorization to market or distribute a medicine is granted through a Notice of Compliance (NOC). A medicine may be temporarily distributed with specified restrictions before receiving an NOC, as an Investigational New Drug or under the Special Access Program.
The PMPRB has no authority to regulate the prices of non-patented drugs, including generic drugs sold under compulsory licenses, and does not have jurisdiction over prices charged by wholesalers or retailers nor over pharmacists' professional fees. Also, matters such as distribution and prescribing are outside the purview of the PMPRB.
Under the Patented Medicines Regulations, patentees are required to file price and sales information twice a year for each strength of each dosage form of each patented medicine sold in Canada for price regulation purposes. Patentees are also required to file R&D expenditures once a year for reporting purposes.
Manufacturers are also required to inform the PMPRB of their intention to sell a new patented medicine but are not required to obtain approval of the price before they do so.
Patentees are required to comply with the Act to ensure that prices of patented medicines sold in Canada are not excessive. In the event that the Board finds, after a public hearing, that a price is excessive in any market it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Reporting - The PMPRB reports annually to Parliament through the Minister of Health. The Annual Report, which covers the calendar year, includes a review of the PMPRB's major activities, analyses of the prices of patented medicines and of the price trends of all drugs, and reports on the R&D expenditures as reported by patent-holding drug manufacturers. In addition, the PMPRB reports through its quarterly NEWSletter and various studies.
Pursuant to an agreement by the Federal/ Provincial/Territorial Ministers of Health and at the request of the federal Minister of Health, the PMPRB conducts research under the National Prescription Drug Utilization Information System (NPDUIS). The purpose of the NPDUIS is to provide critical analyses of price, utilization and cost trends so that Canada's health system has more comprehensive, accurate information on how prescription drugs are being used and on sources of cost increases.
Sales of Drugs in Canada
Total manufacturers' sales of pharmaceuticals for human use are estimated at $15.9 billion in 2004, an increase of 5.3% over sales in 2003. Patentees reported sales of patented medicines of $10.9 billion, an increase of 7.9% over the previous year.
Compliance and Excessive Price Guidelines
Pharmaceutical patentees are required, under section 82 of the Patent Act (Act), to notify the PMPRB of their intention to offer a drug product for sale and the date on which they expect to begin selling it.
Under the Patented Medicines Regulations, 1994 (Regulations), patentees are subsequently required to:
- file a Medicine Identification Sheet (Form 1) within 30 days after either the issuance of a Notice of Compliance or the date on which the drug product was first offered for sale in Canada, whichever comes first;
- report information on the introductory prices and sales of new patented medicines (Form 2) within 60 days of the date of first sale; and
- continue to file detailed information on prices and sales of each patented drug for the first and last six-month period of each year (Form 2) for as long as the drug remains patented.
The PMPRB reviews the pricing information for all patented medicines sold in Canada on an ongoing basis to ensure that the prices charged by patentees comply with the Excessive Price Guidelines (Guidelines) established by the Board. The Guidelines are published in the PMPRB's Compendium of Guidelines, Policies and Procedures (Compendium) and are available on the Web site under Legislation, Regulations, Guidelines, or by calling our toll-free number: 1 877 861-2350.
Excessive Price Guidelines
The Guidelines are based on the price determination factors in section 85 of the Act and have been developed in consultation with stakeholders, including the provincial and territorial Ministers of Health, consumer groups and the pharmaceutical industry. In summary, the Guidelines provide that:
- prices for most new patented drugs are limited such that the cost of therapy for the new drug does not exceed the highest cost of therapy for existing drugs used to treat the same disease in Canada;
- prices of breakthrough patented drugs and those which bring a substantial improvement are generally limited to the median of the prices charged for the same drug in other industrialized countries listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States);
- price increases for existing patented medicines are limited to changes in the Consumer Price Index (CPI); and
- the price of a patented drug in Canada may, at no time, exceed the highest price for the same drug in the foreign countries listed in the Regulations.
Board Staff reviews the prices of all patented medicines sold in Canada. When it finds that the price of a patented drug product appears to exceed the Guidelines, and the circumstances meet the criteria for commencing an investigation, Board Staff will conduct an investigation to determine the facts. Additional information on the criteria for commencing an investigation is available in Annex 1 on page 45. An investigation could result in:
- its closure where it is concluded that the price was within the Guidelines;
- a Voluntary Compliance Undertaking (VCU) by the manufacturer to reduce the price and take other measures to comply with the Guidelines; or
- a public hearing to determine if the price is excessive and to make a remedial order.
As part of the PMPRB's transparency initiative, beginning in 2001, the list of New Patented Medicines Reported to the PMPRB is posted on the PMPRB Web site every month. This list includes information on the status of the review (i.e., under review, within Guidelines, VCU, Notice of Hearing). Drug products "under review" also include drugs which are subject to an investigation. As reported in the April 2005 NEWSletter, beginning in 2005, drugs that are the subject of an investigation will no longer be reported as "under review". When the price appears to exceed the Guidelines and where the criteria for commencing an investigation have been triggered, these drug products will be identified as "under investigation".
New Active Substances in 2004
Health Canada reported 17 New Active Substances (NASs) in 2004 but not all were introduced to the market in that year. [2] The PMPRB's list of patented NASs in any year may differ from the list of NASs approved by Health Canada's Therapeutic Products Directorate (TPD) for the following reasons:
- the NAS is not patented and therefore not subject to the PMPRB's jurisdiction;
- the NAS may not be on the TPD list because it is being sold under the Special Access Program (SAP) before it receives a Notice of Compliance (NOC); or
- the NAS may have been approved, but is not being sold.
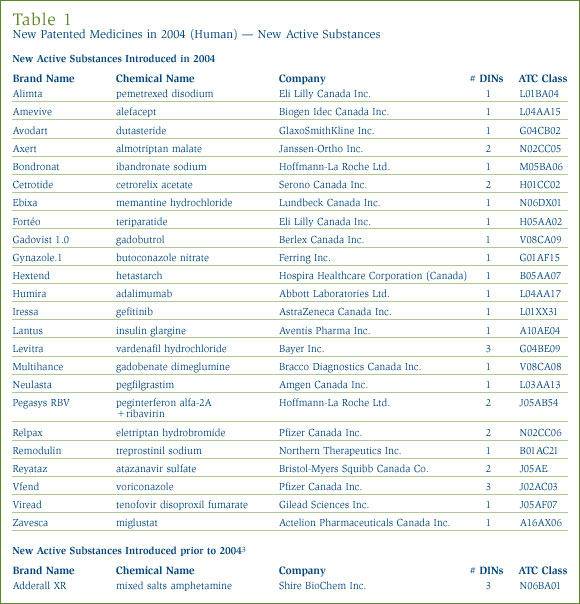
As shown on Table 1 and in Figure 1, one of the 25 patented NASs that came under the PMPRB's jurisdiction was sold prior to 2004.
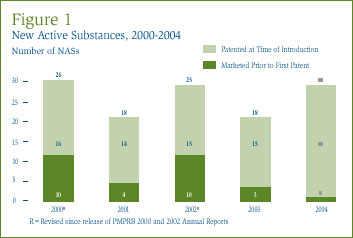
An NAS may include more than one DIN if it is sold in more than one strength or dosage form. The 25 NASs listed for 2004 were marketed as 36 presentations (DINs). Figure 2 provides a breakdown of the patented NASs for human use, by category assigned for price review purposes, over the five-year period 2000 through 2004 inclusive. [4]
Summary Reports of the price reviews of NASs are posted on the PMPRB Web site when completed.
Since last year's Annual Report, Figures 1 and 2 have been updated to include the NAS Dicetel (pinaverium bromide, Solvay Pharma Inc.) for the year 2000. The manufacturer reported this product to the PMPRB in March 2004 and its price is currently under review.
New Patented Drug Products in 2004
There were 94 new patented drug products, or DINs, for human use introduced in 2004. Some are one or more strengths of an NAS and others are new presentations of existing medicines.
For purposes of our price review, any patented drug product introduced in Canada, or previously marketed but first patented, between December 1, 2003 and November 30, 2004, is considered a new patented drug product in 2004. [5]
Six (6.4%) of the 94 new patented DINs were being sold in Canada prior to the issuance of a Canadian patent which brought them under the PMPRB's jurisdiction. These DINs are denoted by a "FPG" (first patent granted) in Annex 2 on page 46. Table 2, on page 12, identifies the number of patented drug products by the year in which they were first sold. The time delay between date of first sale and date of patent grant for these products ranged from several months to two years.

Price Review of New Patented Drugs for Human Use
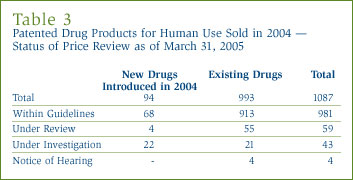
A list of the 94 new patented drug products and their price review status as of March 31, 2005 appears in Annex 2 on page 46. Of the 94 new patented DINs, the prices of 90 had been reviewed. Initially 25 were priced at levels which appeared to be outside the Guidelines and investigations were commenced. At the time of this report, three investigations have been closed - two due to a VCU and the price of one drug product was found to be within the Guidelines, leaving 22 ongoing investigations. For a more detailed explanation of the criteria for commencing an investigation, please refer to Annex 1 on page 45. A total of 68 new patented DINs introduced in 2004 were found to be within the Guidelines.
Price Review of Existing Patented Drugs for Human Use
For the purpose of this report, existing medicines include all patented drug products that were introduced prior to December 1, 2003. The PMPRB's Guidelines limit the price changes for existing patented drugs to changes in the Consumer Price Index (CPI). In addition, the price of a patented drug cannot exceed the highest price of the same drug product in the countries listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, U.K. and U.S.).
A total of 993 existing patented drug products (DINs) for human use were sold during 2004. There were 51 investigations under way at the beginning of the year and, during 2004, investigations were opened into 11 existing patented drug products (DINs) with prices that appeared to be outside the Guidelines. Of the total 62 investigations, 41 were closed leaving 21 investigations into existing drugs ongoing at the end of the year.
At the time of this report:
- the prices of 913 existing DINs (92%) were within the Guidelines;
- 21 DINs were the subject of investigations commenced as a result of pricing in earlier periods (5 are new medicines introduced in 2002, 6 are new medicines introduced in 2003; the remaining 10 are existing medicines - five are from 2003 and five are from 2004);
- 4 DINs, 3 pertaining to Nicoderm and 1 pertaining to Dovobet, were the subject of a hearing under section 83 (see Quasi-judicial Activities on page 18); and
- 55 DINs were still under review.
A summary of the review, compliance and investigation status, as of March 31, 2005, of the new and existing patented drug products for human use in 2004 is provided in Table 3.
Last year we reported that the PMPRB had focused on a number of initiatives internally with respect to the Timelines Review Project resulting in a significant reduction in the number of ongoing investigations from 67 at March 31, 2003 to 51 as of March 31, 2004. Work continued on the Timelines Review Project and further initiatives were implemented in 2004 (refer to page 34). The number of investigations has been further reduced to 43 as of March 31, 2005.
CDR / PMPRB
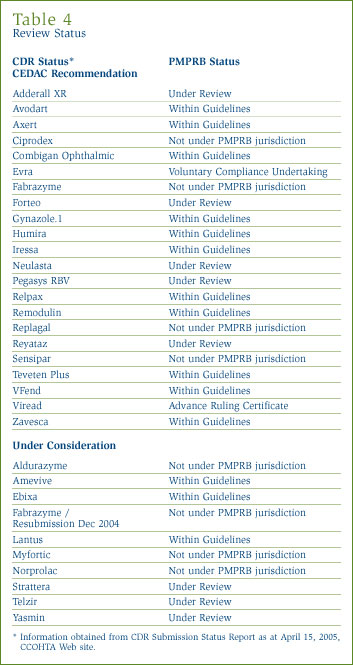
The Common Drug Review (CDR) is a single process for reviewing new drugs and providing formulary listing recommendations to participating publicly-funded federal, provincial and territorial (F/P/T) drug benefit plans in Canada. All jurisdictions are participating except Quebec. The CDR reviews new drugs and provides an evidence-based formulary listing recommendation, made by the Canadian Expert Drug Advisory Committee (CEDAC). The drug plans consider the CEDAC recommendation and also their individual plan mandates, priorities and resources when making formulary listing and coverage decisions. More information on CDR and CEDAC is available from the Canadian Coordinating Office of Health Technology Assessment (CCOHTA) Web site (http://www.ccohta.ca).
Table 4 provides information on CDR reviews and on the PMPRB price reviews.
Update of New Patented Drug Products reported in previous Annual Reports
The following table provides an update as at March 31, 2005 of the new patented medicines, at the DIN level, reported in previous years' Annual Reports.
Update of Existing Medicines from the 2003 Annual Report
In last year's Annual Report, it was reported that of the 974 existing patented drug products for human use sold in 2003, the prices of 41 were still under review. The results of those reviews concluded that 19 had been within the Guidelines; 4 DINs were priced at levels that appeared to exceed the Guidelines and therefore investigations were opened. Eighteen are still under review and included in the total figure of under review reported in Table 3, on page 12.
In its 2003 Report, the PMPRB had also reported that 39 DINs were under investigation. Of those, 34 investigations have been concluded: in 28 cases the prices were ultimately found to be within the Guidelines; and for 6 cases, a Voluntary Compliance Undertaking was approved - Fasturtec, One-Alpha, Tamiflu, Starnoc, Busulfex and Prolastin. (See Voluntary Compliance Undertakings on page 15).
Patented Drugs for Veterinary Use
In September 2003, the PMPRB decided to implement a full complaints-driven approach for the regulation of patented veterinary drug prices. This decision was communicated to stakeholders in the PMPRB's January 2004 NEWSletter. Since the Regulations do not distinguish between the filing requirements for human drug patentees and veterinary drug patentees, they need to be amended to differentiate between the filing requirements of the two. Proposed amendments to the Regulations for consultation with stakeholders were published in the January 2005 NEWSletter. The deadline for stakeholders' submissions on the proposed amendments to the Regulations was April 15, 2005. The Board will report next steps in this initiative and publish stakeholders' submissions, through its Research Agenda.
For the time being, the complaints-driven approach for regulating the prices of patented veterinary drugs remains in place. Board Staff reviews the prices of new patented medicines only. Existing medicines are subject to review only when a substantiated complaint has been received. No complaints were received in 2004.
In last year's Annual Report it was reported that seven DINs were under review. Five of those have been found to be within the Guidelines and the remaining two, plus the additional one introduced in 2003, are still under review. The summary reports of the price review of veterinary drug products are made available on the PMPRB's Web site under Other Publications; Patented Medicines; Reports on New Patented Drugs for Veterinary Use.
An Advance Ruling Certificate (ARC) is non-binding and may be issued pursuant to subsection 98(4) of the Patent Act at the request of a patentee when the Board is satisfied that the price or proposed price of the medicine would not exceed the maximum non-excessive price under the PMPRB's Price Guidelines. In accordance with subsection 98(4) of the Act, any review conducted for the purposes of issuing an ARC is based on the material facts available at the time of the review.
Viread, Gilead Sciences, Inc.
On April 13, 2004, the Board chose, in this case, to publish a Notice and Comment proposing to issue an Advance Ruling Certificate (ARC) with respect to the patented medicine Viread.
Viread is used in the treatment of HIV-1 infection and has been sold in Canada by Gilead Sciences, Inc. since March 2004.
Following negotiations, Gilead and Board Staff agreed that Gilead would propose to sell Viread in Canada at an average price not to exceed $15.1250 per 300 mg tablet.
Board Staff recommended that it was appropriate for the Board to conclude that it would not have sufficient grounds to make an order under section 83 of the Act with respect to Viread, taking into consideration the factors set out in section 85 of the Act.
The Board received one submission in response to its Notice and Comment from the Canadian Treatment Action Council (CTAC). There were no submissions from the Ministers of Health in the provinces and territories. Gilead and Board Staff filed written submissions in response to the submission from CTAC.
Having considered the submissions of CTAC, Gilead and Board Staff, and based on the facts available at the time, the Chairperson concluded that there would not be sufficient grounds to make an order under section 83 of the Act and that it was in the public interest to issue the ARC with respect to the proposed price of the medicine Viread. The PMPRB will continue to monitor the price of Viread to ensure that it complies with the Board's Guidelines while it remains under the PMPRB's jurisdiction.
The Advance Ruling Certificate and Board Reasons are published on our Web site under Publications; Advance Ruling Certificates; Viread. Copies of submissions filed may be obtained from the Secretary of the Board.
Under the Compliance and Enforcement Policy, patentees are given an opportunity to submit a VCU when Board Staff concludes, following an investigation, that the price set forth by the patentee appears to have exceeded the PMPRB's Price Guidelines.
A Voluntary Compliance Undertaking (VCU) is a written undertaking by a patentee to adjust its price to conform with the PMPRB's Excessive Price Guidelines. Detailed information and definitions are available in the Glossary Section of the Report.
Acceptance of a VCU by the Chairperson is an alternative to the commencement of formal proceedings through the issuance of a Notice of Hearing. Under the PMPRB's Compliance and Enforcement Policy, a VCU can also be submitted following the issuance of a Notice of Hearing. A VCU submitted at this point must be approved by the Board.
In 2004, five VCUs were approved for the patented medicines:
- One-Alpha, LEO Pharma Inc.
- Fasturtec, Sanofi-Synthelabo Canada Inc.
- Prolastin, Bayer Inc.
- Starnoc, Servier Canada Inc.
- Busulfex, ESP Pharma Inc.
In February 2005, the Board also accepted a VCU submitted for the patented medicine Evra by Janssen-Ortho Inc. The Chairperson accepted two other VCUs in March 2005, for the patented medicines Paxil CR, submitted by GlaxoSmithKline Inc., and Tamiflu, submitted by Hoffmann-La Roche Limited.
One-Alpha, LEO Pharma Inc.
One-Alpha is indicated for the management of hypocalcaemia, secondary hyperparathyroidism and osteodystrophy in patients with chronic renal failure.
On May 6, 2004, the Chairperson approved a VCU from LEO Pharma Inc. for the patented drug One-Alpha (alfacalcidol). LEO Pharma began selling One-Alpha in January 2001.
Under the terms and conditions of the VCU, LEO Pharma reduced the average selling price of One-Alpha so that the average price for 2004 did not exceed the 2004 maximum non-excessive (MNE) price of $13.3750 per ml. To offset excess revenues received during the period of January 1, 2001 to December 31, 2003, LEO Pharma made a payment to the Government of Canada in the amount of $23,049.10.
Further information concerning the VCU from LEO Pharma for One-Alpha was published in our 2003 Annual Report. The VCU is also available on our Web site under Publications; Voluntary Compliance Undertakings.
Fasturtec, Sanofi-Synthelabo Canada Inc.
Fasturtec is indicated for the treatment and prevention of hyperuricemia in paediatric and adult cancer patients. It is administered intravenously in a hospital setting.
The Board concluded proceedings commenced on May 20, 2004, in regard to the patented medicine Fasturtec, by accepting a VCU by Sanofi-Synthelabo Canada Inc. (Sanofi). Under the terms of the VCU, Sanofi lowered the price of Fasturtec from $295.00 per vial to about $125.00 per vial, effective by July 26, 2004.
Among other things, the VCU benefited Canadian consumers and the healthcare system by immediately reducing the price of Fasturtec to less than half the average selling price and ensures that no customer in Canada shall pay a price higher than the MNE price in the future. Under the terms of the VCU, the price is within the PMPRB Guidelines and will remain so while it is under the Board's jurisdiction, at least until 2015.
The terms of the VCU require that the average selling price for 2004 not exceed the MNE price of $124.7854. Furthermore, Sanofi also offset excess revenues received from May 21, 2002 to December 31, 2003, by providing rebates directly to each of its customers, 28 hospitals that purchased Fasturtec over this period at the higher price.
In its submission, Board Staff noted that Sanofi intends to maintain a list price for Fasturtec which is substantially higher than the reduced price, despite its undertaking that no customer in Canada would pay a price higher than the reduced price. The Board initiated a review of this issue. Further information on the Board's initiative is available under Policy and Research Initiatives on page 35.
Prolastin, Bayer Inc.
Prolastin is a drug product derived from human plasma. It is indicated for a rare genetic disorder, specifically, chronic replacement therapy of individuals having congenital deficiency of alpha 1-PI (alpha 1-antitrypsin deficiency) with clinically demonstrable panacinar emphysema.
On July 9, the Chairperson accepted a VCU submitted by Bayer Inc. with respect to the price of the patented drug Prolastin.
The terms of the VCU required that for purposes of the PMPRB's Price Guidelines, the MNE price of Prolastin in 2003 be $288.00 and the average transaction price of Prolastin in 2003 not exceed $288.00 per vial.
Bayer also undertook to sell Prolastin in Canada during 2004, 2005 and 2006 at a price that will not exceed the lower of (a) the $288.00 MNE price in 2003 adjusted for CPI increases in 2004, 2005 and 2006 and (b) the median international prices in those years. In the event that Bayer proposes to increase the price of Prolastin in any succeeding year after 2006 by more than its CPI-adjusted price as determined in accordance with the methodology established in the Guidelines, it further undertook to provide written notification to the PMPRB and satisfactory written evidence in support of the rationale for any such price increase. In light of the particular circumstances of this case, the Chairperson accepted the VCU. The PMPRB reserves its right to commence an investigation in appropriate circumstances pursuant to its Compliance and Enforcement Policy.
Starnoc, Servier Canada Inc.
Starnoc is indicated for the short-term treatment and symptomatic relief of insomnia in patients who have difficulty falling asleep.
The PMPRB concluded an investigation into the price of the patented drug Starnoc, resulting in a VCU to reduce its price by more than 40%, and to reimburse excess revenues.
On July 15, the Chairperson accepted a VCU submitted by Servier Canada Inc., the terms of which required that for the purposes of the PMPRB's Price Guidelines, the MNE prices of Starnoc 5 mg capsule in Canada for 2000 and 2004 are $0.4526 and $0.4964, respectively while the MNE prices of Starnoc 10 mg capsule in Canada for 2000 and 2004 are $0.6816 and $0.7475, respectively.
To ensure that the prices of Starnoc in 2004 were within the Guidelines, Servier made a payment of $739,739.99 to the Government of Canada to offset excess revenues received during the period from January 1, 2004 to June 30, 2004.
To offset the remaining excess revenues of $3,838,801.86, Servier will maintain the prices of all of its patented medicines at levels below the CPI-adjusted prices until the end of 2005. In the event that any excess revenues have not been offset by the end of December 2005, Servier has undertaken to make a payment to the Government by January 30, 2006 for such amount.
The prices of Starnoc will remain under the PMPRB's jurisdiction until the expiry of the patent in June 2007.
Busulfex, ESP Pharma Inc.
Busulfex is an antineoplastic agent indicated for use in combination with other chemotherapeutic agents and/or radiotherapy as a conditioning regimen prior to hematopoietic progenitor cell transplantation (HPCT), or bone marrow transplant.
The PMPRB concluded an investigation into the price of the patented drug Busulfex, resulting in a price reduction of over 7%.
On November 16, the Chairperson accepted a VCU by ESP Pharma. The terms of this VCU required that, for the purposes of complying with the PMPRB's Price Guidelines, ESP lower the average transaction price of Busulfex to the 2004 MNE price of $359.89 per ampoule. To offset excess revenues received, ESP made payments totaling $150,646.99 to the Government of Canada.
Busulfex has been sold in Canada since April 1999. The patent pertaining to Busulfex was granted in July 2002. Although Busulfex came under the PMPRB's jurisdiction in July 2002, the PMPRB's price review jurisdiction extends retroactively to include pre-grant patent infringement period, which in this instance is April 1999.
The price of Busulfex will remain under the PMPRB's jurisdiction until the expiry of the patent in August 2014.
Evra, Janssen-Ortho Inc.
Evra is a transdermal contraceptive patch indicated for the prevention of pregnancy in women who elect to use hormonal contraceptives.
On February 21, 2005, the Board concluded proceedings commenced on December 23, 2004, in regard to the patented medicine Evra by accepting a VCU by Janssen-Ortho Inc. Under the terms of the VCU, Janssen-Ortho lowered the price of Evra by approximately 45% to $4.47 per patch.
To offset excess revenues from past sales of Evra accrued from the date of first sale to June 30, 2004, Janssen-Ortho made a payment to the Government of Canada in the amount of $1,359,263.67. Finally, the balance of excess revenues remaining, totalling $1,496,019.02, for the period July 1, 2004 to December 31, 2004, will be offset by reducing the price of one of Janssen-Ortho's patented medicines, Levaquin 5mg/mL and 25mg/mL as of March 1, 2005.
In the event that the full amount of the excess revenues is not offset by December 31, 2005, Janssen-Ortho will offset any remaining excess revenues by making a further payment to the Government of Canada no later than January 31, 2006.
The price of Evra will remain under the PMPRB's jurisdiction until the expiry of the patent in June 2016.
Paxil CR, GlaxoSmithKline Inc.
Paxil CR provides a controlled-release to the alternative range of presentations of Paxil, an anti-depressant. It is supplied in the form of tablets in two strengths, 12.5 mg tablet and 25 mg tablet.
In order to comply with the PMPRB's Price Guidelines, GlaxoSmithKline (GSK) undertook to reduce the average transaction price of Paxil CR by the end of the January 1 to June 30, 2005 regulatory filing period such that the average transaction price for 2005 does not exceed the 2005 MNE price of $1.5861 for Paxil CR 12.5 mg and $1.7019 for Paxil CR 25 mg.
GSK offset excess revenues it received during the period of January 5, 2004 to December 31, 2004 by making a payment to the Government of Canada in the amount of $310,403.64.
The price of Paxil CR will remain under the PMPRB's jurisdiction until the expiry of the patent in July 2016.
Tamiflu, Hoffmann-La Roche Limited
Tamiflu is a direct acting antiviral neuraminidase inhibitor.
In order to comply with the PMPRB's Price Guidelines, Hoffmann-La Roche Limited (Roche) agreed that the MNE price of Tamiflu 75 mg capsule was $3.7695 for the period January to December 2003; $3.8383 for the period January to December 2004; and is $3.8917 for the period January to December 2005. Roche undertook to ensure that the average transaction price of Tamiflu 75 mg capsule does not exceed the MNE price of $3.8917 per capsule for 2005. Also, Roche offset excess revenues received for the reporting periods January 1, 2003 to December 31, 2004 by making a payment to the Government of Canada in the amount of $442,973.47.
The price of Tamiflu will remain under the PMPRB's jurisdiction until the expiry of the patent in May 2019.
Nicoderm, Hoechst Marion Roussel Canada Inc.
Nicoderm is a transdermal nicotine patch, indicated as an aid for smoking cessation for the partial relief of nicotine withdrawal symptoms.
On April 20, 1999, the Board issued a Notice of Hearing to consider whether, under sections 83 and 85 of the Patent Act, Nicoderm is being, or has been, sold by Hoechst Marion Roussel Canada Inc. (HMRC) in Canada at a price that, in the opinion of the Board, is excessive and if so, what order, if any, should be made. The matter was reported on in previous Annual Reports and selected issues of the NEWSletter.
Following the issuance of the Board's decisions in 1999 and 2000 affirming its jurisdiction to conduct a hearing into the price of Nicoderm, HMRC commenced two judicial review applications in the Federal Court of Canada seeking to set aside the Board's decisions. The Federal Court heard the judicial review applications in this matter on May 16-17, 2005. Decisions are pending.
Fasturtec, Sanofi-Synthelabo Canada Inc.
Fasturtec is indicated for the treatment and prevention of hyperuricemia in paediatric and adult cancer patients. It is administered intravenously in a hospital setting.
On June 28, 2004, the Board concluded proceedings commenced in May in regard to the medicine Fasturtec by accepting a Voluntary Compliance Undertaking by Sanofi-Synthelabo Canada Inc. Under the terms of the VCU, Sanofi lowered the price of Fasturtec from $295.00 per vial to about $125.00 per vial, effective by July 26, 2004.
Details on this matter appear in the Voluntary Compliance Undertaking section of this report on page 16.
Dovobet, LEO Pharma Inc.
Dovobet is indicated for the topical treatment of active lesions of psoriasis vulgaris in adult patients.
The Board issued a Notice of Hearing on November 29, 2004 in the matter of LEO Pharma Inc. and the price of the patented medicine Dovobet. The purpose of the hearing is to determine whether, under sections 83 and 85 of the Patent Act, LEO Pharma Inc. is selling or has sold the medicine known as Dovobet in any market in Canada at a price that, in the Board's opinion, is or was excessive; and, if so, what order, if any, should be made.
The Board held a pre-hearing conference on January 19, 2005 and three days of hearing in March. The Board resumes sitting on the merits of this case in September.
Evra, Janssen-Ortho Inc.
Evra is a transdermal contraceptive patch indicated for the prevention of pregnancy in women who elect to use hormonal contraceptives.
On December 23, 2004, the Board issued a Notice of Hearing in the matter of Janssen-Ortho Inc. and the price of the patented medicine Evra. On February 21, 2005, the Board approved a VCU to reduce the price of Evra.
The terms of the VCU required Janssen-Ortho to lower the price of Evra by 45% to $4.47 per patch, to make a payment to the Government of Canada and to lower the price of another of its patented medicines, Levaquin, in order to offset excess revenues from past sales of Evra.
Details on this matter appear in the Voluntary Compliance Undertaking section of this report on page 17.
This section of the Annual Report has been re-organized and new tables and figures have been added. It is hoped that the more in-depth analysis of the key indices will assist the readers in better understanding the current context. These changes are very much in line with the reporting mandate of the PMPRB to contribute to informed decisions and policy making by reporting, among others, on pharmaceutical trends.
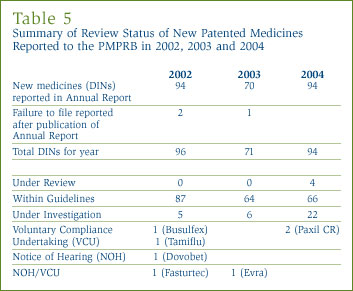
Pharmaceutical sales rose to $15.9 billion in 2004, a 5.3% increase over 2003 sales. [6] This rate of growth is markedly less than the 15.2% recorded in the previous year, and was in fact the lowest rate seen since 1996. Table 6 gives the estimated amount of total manufacturers' sales of drugs in Canada for the years 1990 through 2004.
Sales and Price: Rising levels of drug sales do not as such imply rising drug prices, or the reverse. [7] Several other factors - affecting the volume and composition of drug utilization - can produce high rates of sales growth even where drug prices are falling on average. These include: [8]
- changes in total population;
- changes in the demographic composition of the population (e.g., movement in age-distribution towards older persons with more health problems);
- increased incidence (within particular demographic groups) of health problems calling for drug therapy;
- changes in the prescribing habits of physicians (e.g., shifts away from older, less expensive drugs to newer, more expensive medications treating the same condition, possibly more effectively);
- greater use of drug therapy rather than other treatments (e.g., instead of surgery);
- use of innovative drug therapies to treat conditions where no effective treatment existed previously; and,
- greater propensity on the part of physicians and patients (e.g., in response to new medical findings) to use drugs to treat conditions not previously considered problematic.
The Composition of Manufacturers' Sales: The PMPRB estimates total manufacturers' sales by summing sales of patented, non-patented brand name and generic drugs. For this purpose, a "patented drug" is any product currently subject to PMPRB price review. A "non-patented brand name drug" is a product sold by a current patentee (that is, a manufacturer currently selling one or more products subject to PMPRB price review) that is not itself currently patented (either because a patent is pending, all patents applicable to the product have expired or because the product was never patented).
Patentees are required, under the Patented Medicines Regulations, to report their total sales of drugs in Canada, both patented and non-patented, to the PMPRB. Patentees are also required to submit detailed information, by product and class of purchaser, on their sales of currently patented drugs. This information allows the PMPRB to directly calculate sales of patented drugs for each patentee, and to infer the amount of each patentee's total drug sales attributable to non-patented drugs. To complete its calculations, the PMPRB obtains estimates of generic sales by members of the Canadian Generic Pharmaceutical Association (CGPA) from IMS Health. [9]
Returning to Table 6, on page 19, sales of patented drugs rose to $10.9 billion in 2004, an increase of 7.9% over the corresponding 2003 value. This was the smallest rate of increase seen since 1994. Patented drugs accounted for 68.6% of total drug sales in 2004, slightly more than in the previous year. The share of patented drugs in total manufacturers' sales has risen markedly since the mid-1990s, when patented drugs accounted for less than half of total sales.
Figure 3 provides further information on the composition of manufacturers' sales. The decline in the relative importance of non-patented brand name drugs is particularly remarkable. In 1995 non-patented brand name drugs accounted for nearly half of the total drug sales. With almost no growth in this component of sales, the non-patented brand name share has declined steadily since then, reaching 18.9% in 2004. In contrast, the share of generic products has risen over the same period, standing at 10.0% in 1995 and 12.5% in 2004. Generic sales have more than tripled over this period, from $0.6 billion to $2.0 billion.
Why the dramatic decline in the growth of patented drug sales in 2004? Several leading products went off patent in 2004, while other patented products have continued to lose market-share to non-patented competitors. Product withdrawals (mostly restricted to the second half of 2004) also contributed, albeit in a relatively small way, to the decline in sales growth.
There are also longer-term factors at work here. Throughout the 1990s growth in sales of patented drugs was largely driven by a succession of new, high-selling "blockbuster" products. Since the beginning of the current decade, the pharmaceutical industry has not introduced new "blockbusters" to market in sufficient numbers to sustain the high rates of growth seen in the 1990s. Products introduced since 2000 have contributed much less in the way of additional sales than did the new products of the late 1990s, both in absolute terms and relative to the sales base (which, in the case of patented drugs, is now more than twice what it was in 1999). Slowing of the growth of patented medicine sales was masked in the first few years of this decade by the still-growing sales of "blockbuster" products introduced in the late 1990s.
Sales by Therapeutic Class: For purposes of price review, the PMPRB classifies drugs using the World Health Organization's (WHO) Anatomical Therapeutic Chemical (ATC) classification system. This is a hierarchical system that classifies drugs according to their principle therapeutic uses and chemical composition. At its highest level, Level I, the ATC system classifies drugs according to the aspect of human anatomy with which they are principally associated.
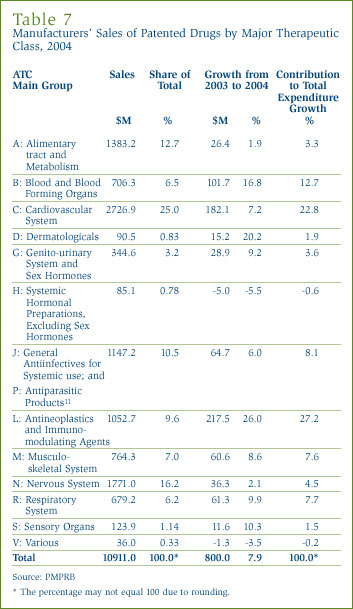
Table 7 breaks out the manufacturers' sales of patented drugs in Canada in 2004 by major therapeutic class, defined by the set of Level I ATC classes. [10] The table lists the 2004 sales for each class, the share of overall sales this represents and the rate of increase/decrease in sales relative to 2003 sales. The last column in this table gives the ratio of each class' sales increase to the overall increase in sales of patented drugs, that is, each class' "contribution" to the increase in overall sales. The values in this column thus indicate which classes were the primary drivers of overall sales growth. In 2004 these primary drivers included:
- antineoplastics and immunomodulating agents (accounting for 27.2% of the increase in overall sales of patented drugs);
- drugs related to the cardiovascular system, such as lipid-reducing agents and drugs treating hypertension (22.8%);
- drugs related to blood and blood forming organs (12.7%); and
- general antiinfectives and antiparasitic products (8.1%).
These four classes accounted for almost three quarters of the growth in manufacturers' sales between 2003 and 2004. Cardiovascular drugs have been a leading driver of sales growth for many years. The leading contribution of antineoplastics and immunomodulating agents (for the most part drugs used in chemotherapy) is unprecedented. Note as well that several therapeutic classes that have emerged as major drivers in past years, such as drugs related to the alimentary tract and metabolism, the nervous system and the respiratory system, contributed little to sales growth in 2004.
The PMPRB maintains the Patented Medicine Price Index (PMPI) to monitor trends in prices of patented drugs. The PMPI measures average year-over-year changes in the ex-factory prices of patented drug products sold in Canada. It is updated annually using price and sales information reported by patentees. [12]
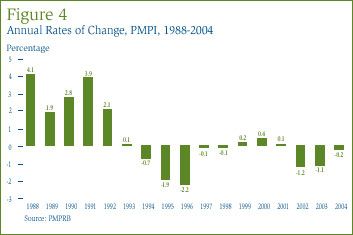
Figure 4 provides year-over-year changes in the PMPI for the years 1988 through 2004. As measured by the PMPI, manufacturers' prices of patented drugs fell on average by 0.2% in 2004. This result continues a pattern of declines and near-negligible increases that began in 1993. As in previous years, the price stability observed in 2004 was broadly based, with a majority of patented drug prices exhibiting little or no change.
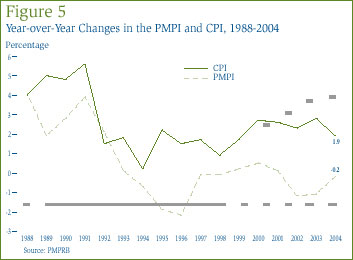
Comparison of PMPI and CPI: The Patent Act provides that, among other factors, the PMPRB shall consider changes in the Consumer Price Index (CPI) in determining whether the price of a patented drug is excessive. Figure 5 plots year-over-year rates of change in the PMPI against corresponding changes in the CPI. General price inflation, as measured by the CPI, has exceeded the average increase in patented drug prices almost every year since 1988. [13] This occurred again in 2004, with CPI-inflation exceeding the rate of PMPI change by approximately 2.1% [14], although the PMPI-CPI gap narrowed considerably between 2003 and 2004.
That the PMPI has consistently risen less rapidly than the CPI is not surprising. The PMPRB's Guidelines require price increases over any three-year period to be no greater than CPI-inflation. In addition, they impose a cap on year-over-year price increases equal to one-and-one half times the rate of CPI-inflation for the year in question. These requirements, applied to patented drugs product-by-product, have the effect of establishing CPI-inflation as an upper bound on increases in the PMPI. Furthermore, movements in the PMPI have never attained this limit because some manufacturers either do not raise their prices by the full amount permitted under the PMPRB's Guidelines or reduce their prices.
Figure 6 provides information on the extent to which manufacturers have taken the increases permitted under PMPRB Guidelines. In 2004, 52% of patented drug prices rose by between zero and the allowable maximum, compared to only 38% in 2001. Restricting the analysis to the 200 highest-selling drugs in 2004, 66% of patented drugs took price increases within the allowable maximum, compared to only 41% in 2001.
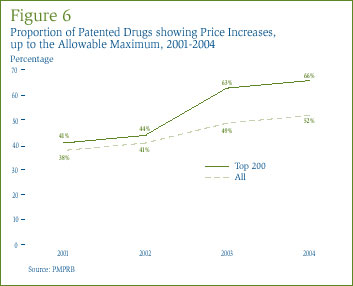
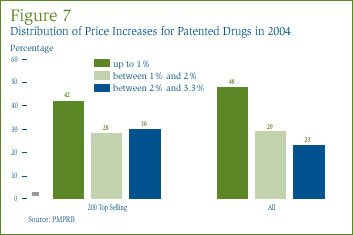
Figure 7 provides information on the distribution of price increases. In 2004, 48% of patented drug prices rose by 1% while 52% rose between 1% and 3.3%. Restricting the analysis to the 200 highest-selling drugs, 42% of patented drug prices rose by 1% while 58% rose between 1% and 3.3%. The Guidelines provide that one year price increases cannot exceed 1.5 times the forecast change in CPI. For 2004, the maximum allowable price increase was 3.3%.
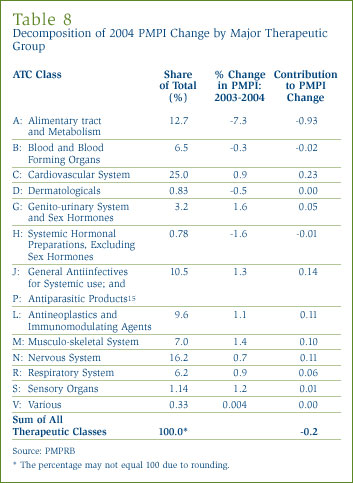
Price Change by Therapeutic Class: Table 8, on page 24, provides average rates of price change among patented drugs at the level of major therapeutic classes. The results in this table were obtained by applying the PMPI methodology to data on prices among all patented drugs within a given class. The table lists each class' share of overall patented drug sales, as well as average rate of price change specific to the class. The last column multiplies each class' average rate of price change by its share of overall sales: the resulting value equals the group's "contribution" to the change in the overall PMPI (as depicted in Figure 7). The values in this column thus indicate which classes were the primary drivers of the overall price change in the entire set of patented drugs.
The results of Table 8 illustrate the inadvisability of relying too heavily on a single, comprehensive measure of price change. It is clear that several leading therapeutic classes saw price increases between 2003 and 2004, despite the drop in the PMPI. It is also clear that the single largest influence on the PMPI was the decline of prices among drugs related to the alimentary tract and metabolism (ATC Class A). The contribution of this one class more than offset the contributions of all others: without the price changes observed in Class A, the PMPI would have risen by some 0.7%.
Throughout 2004, the PMPRB had been advised of a number of reported price increases for patented drugs. Due to the number of price increases reported in 2004, the PMPRB initiated a consultation with stakeholders on the issue of price increases for patented medicines. A dialogue with stakeholders on this issue began with the publication of the PMPRB discussion paper on March 8, 2005. Further analysis on the issue of price increases for patented medicines will be conducted in 2005. The PMPRB will continue to report on this policy review through its Research Agenda.
In accordance with the Patent Act and the Patented Medicines Regulations, patentees must report all publicly available ex-factory prices of patented drugs in seven foreign countries: France, Germany, Italy, Sweden, Switzerland, the U.K. and the U.S. The PMPRB uses this foreign price information to:
- conduct the International Price Comparison (IPC) tests specified in the Guidelines; and,
- compare drug prices in Canada with other countries.
Figure 8 shows the average ratio of Canadian prices to the median of prices among the seven comparator countries (the "median international price") over the years 1987 through 2004. [16] Canadian prices were on average 23% higher than the median international price in 1987. The average ratio declined to 0.93 in 1995, remaining at levels 5% to 12% below parity from 1995 to 2001. After rising to 1.01 in 2002, the average ratio is again well below parity: in 2004 the average ratio was 0.91.
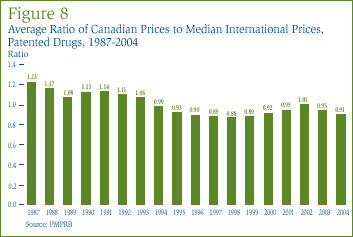
The ratios presented in Figure 8 are sales-weighted averages of the ratio of the Canadian price to the median international price for each patented drug product for which patentees have reported one or more foreign prices. A key step in its calculation is the conversion of foreign prices in local currencies to their Canadian-dollar equivalents. [17] Year-to-year changes in the average ratio can thus reflect:
- trends in Canadian prices;
- trends in international prices;
- exchange rate movements;
- changes in the set of drug products covered (as new patented drugs are introduced to Canada and older drugs go off patent); and
- shifts in revenue shares among drug products.
Sensitivity analysis indicates that exchange rate movements - in particular, an appreciation of the Swedish kroner against the Canadian dollar [18] - account for roughly three-quarters of the decline in the average ratio between 2003 and 2004. Rising foreign prices and shifts in sales-weights account for the remainder. Movements in Canadian prices contributed almost nothing to the average ratio's decline.
Figure 9 shows the relationship between Canadian prices for patented drug products and prices in each of the seven comparator countries. In 1987 Canadian prices were, on average below U.S. prices, but above those in all other countries. By the mid-1990s the situation had changed dramatically, with Canadian prices in the mid-range of the six European countries. This situation continued in 2004, with Canadian prices of patented drugs being on average somewhat less than those in Sweden, Germany, the U.K. and Switzerland, but greater than prices in France and Italy. As in previous years, U.S. prices [19] appear to be substantially higher than prices in both Europe and Canada. The gap is widening.
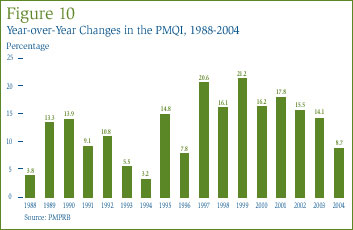
In the context of pharmaceuticals, the concept of "utilization" normally refers to the physical quantities of drugs sold or consumed. The price and sales data used to calculate the PMPI also allow the PMPRB to examine trends in the utilization of patented drugs in Canada. The PMPRB maintains the Patented Medicine Quantity Index (PMQI) for this purpose. [20] Figure 10 displays average rates of utilization growth, as measured by the PMQI, over the years 1988 through 2004. These results confirm that growth in the utilization of patented drugs has been the primary source of rising sales, with rates of utilization growth roughly tracking rates of sales growth in recent years. This pattern continued in 2004: utilization of patented drugs grew by 8.7%, the lowest rate observed since 1996.
Utilization Growth by Class: Table 9 provides average rates of utilization growth among patented drugs at the level of major therapeutic classes. The results in this table were obtained by applying the PMQI methodology to data on physical quantity sold for all patented drugs within a given class. The table lists each class' share of overall patented drug sales, as well as average rate of utilization growth specific to the class. The last column multiplies each class' average rate of utilization growth by its share of overall sales: the resulting value equals the group's "contribution" to the change in the overall PMQI. The values in this column thus indicate which classes were the primary drivers of the overall utilization growth in the entire set of patented drugs. These primary drivers included:
- drugs related to the cardiovascular system (accounting for 2.25% of the overall increase in the PMQI);
- drugs related to the musculo-skeletal system (2.19%); and
- antineoplastics and immunomodulating agents (1.28%).
The global drug industry is dominated by a number of large multinational enterprises based in several countries. Most of these companies have Canadian subsidiaries which, along with a few Canadian-based manufacturers, account for the manufacture, sale and distribution of drugs in Canada.
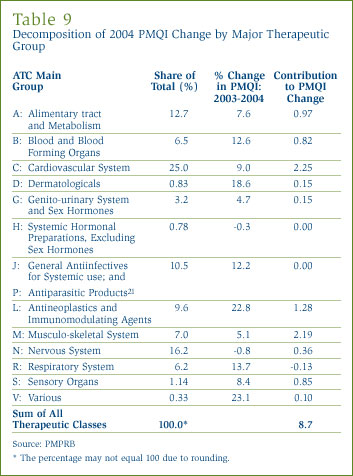
According to Statistics Canada, shipments by Canadian drug manufacturers amounted to $7.8 billion in 2003 accounting for 1.4% of total shipments in the manufacturing sector. Exports account for about $3.4 billion of these shipments, implying that Canadian plants supplied only about $4.4 billion of the $15.1 billion in total drug sales the PMPRB estimates for 2003. The import share becomes higher with the calculation restricted to brand name products (since most generic drugs sold in Canada are produced domestically). [22] It employed 29,312 persons, accounting for 1.5% of total employment in manufacturing. [23] Figure 11 provides year-over-year rates of change in total shipments and employment in drug manufacturing. So far in this decade, growth of both production and employment in drug manufacturing has exceeded growth in the overall manufacturing sector by a considerable margin.
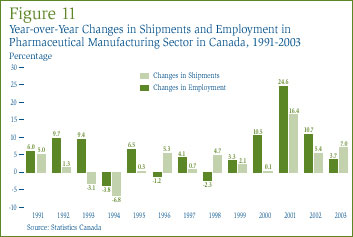
It is of interest in this context to examine recent trends in the prices at which Canadian-manufactured drug products sell. Statistics Canada's IPPI (Pharma) is designed for this purpose. [24] Figure 12 plots year-over-year rates of change in the IPPI (Pharma) for the years 1984 through 2004. By and large, the prices included by the IPPI (Pharma) have not kept pace with overall price inflation in Canada. Rate of increase in the IPPI (Pharma) declined steadily between 1984 and 1993. The index remained virtually unchanged from 1993 to 2001. The index rose by 0.7% in 2004.
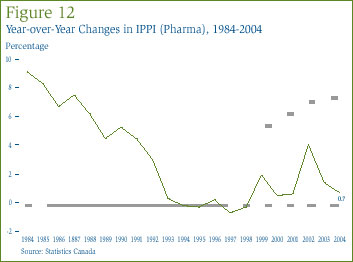
IMS Health regularly reports on manufacturers' sales to the retail sector across a wide range of countries. It reports that in 2004 such sales amounted to $452.3 billion across major markets. [25] Figure 13 shows how this amount was distributed among these markets. Drug sales in Canada accounted for 2.9% of total major-market sales. The U.S. market is by far the largest in the world, with drug sales exceeding the combined sales of Canada, France, Germany, Italy, Japan and the U.K.
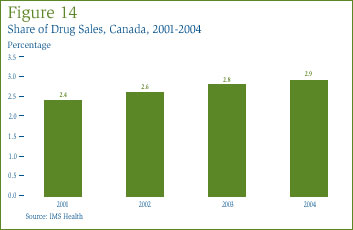
Figure 14 shows Canada's share of major-market sales for each of the years 2001 through 2004. This share has risen steadily since 2001, reflecting the fact that sales growth in Canada has consistently outpaced sales growth in other major markets.
As shown in Figure 15, this pattern continued in 2004, where year-over-year sales growth in Canada (9%) [26] was half-again as great as growth in other major markets (6%).
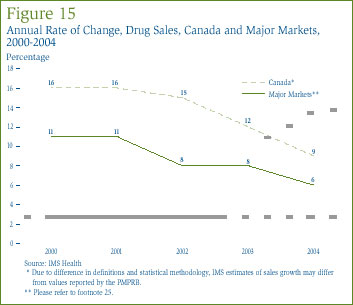
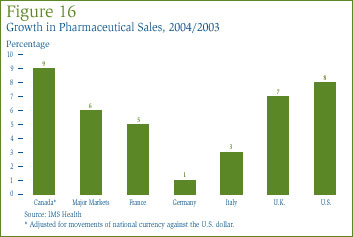
Figure 16, which gives sales growth for individual major markets, shows that while Canadian sales growth was roughly in line with that observed in the U.S., it was far greater than that seen in France, German and Italy.
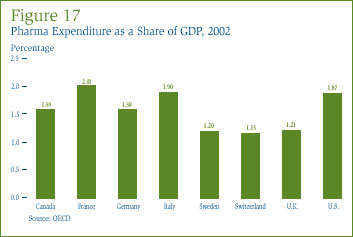
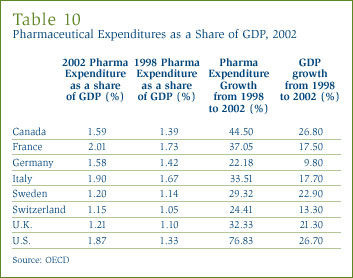
A useful way to compare drug costs internationally is to ask how much of a country's national income is absorbed by pharmaceutical expenditures [27] by its residents. To that end, Figure 17 gives drug expenditures as a share of Gross Domestic Product (GDP) in Canada and seven other countries for 2002. The figure shows that drug expenditures absorbed between 1.2% and 2.0% of GDP in these eight countries. Canada's expenditure-to-GDP ratio, at 1.6%, was in the middle of this range, greater than ratios prevailing in Sweden, Switzerland and the U.K., but somewhat less than those in France, U.S. and Italy.
The share of national income absorbed by pharmaceutical expenditures has increased appreciably in most developed countries over the last decade. Table 10 shows that between 1998 and 2002, with the possible exception of Sweden (i.e. 29.3% pharma expenditure growth versus 22.9% GDP growth), pharmaceutical expenditures grew much more rapidly than GDP in each of the countries represented in Figure 17. The U.S. provides the most striking case in this respect: here pharmaceutical expenditures grew at nearly three times the rate of national income (i.e. 76.8% pharma expenditure growth versus 26.7% GDP growth).
The National Prescription Drug Utilization Information System (NPDUIS) provides critical analyses of price, utilization and cost trends so that Canada's health system has more comprehensive, accurate information on how prescription drugs are being used and on sources of cost increases. The Canadian Institute for Health Information (CIHI) and the PMPRB are partners in the NPDUIS. A steering committee comprising representatives of public drug plans and Health Canada advises CIHI and the PMPRB on the development of the NPDUIS databases and analysis.
The NPDUIS initiative involves two major elements:
- development and implementation of a prescription claims level drug database capable of incorporating program data from publicly-funded drug plans; and
- production of analytical reports relying on information in this database.
CIHI is responsible for the first of these elements, while as per the request of the Minister of Health, the PMPRB is principally responsible for the second.
In September 2004 First Ministers came together and agreed on a 10-year plan to make health care more responsive and sustainable. Among other things, they agreed that no Canadian should suffer undue financial hardship in accessing needed drug therapies. To this end, they directed Health Ministers to establish a Ministerial Task Force (Task Force) to develop and implement the national pharmaceuticals strategy (NPS) and report on progress by June 30, 2006. The strategy will cover a number of initiatives. Of particular relevance to the NPDUIS will be work under the NPS to enhance analysis of cost drivers and cost-effectiveness, including best practices in drug plan policies.
The NPS work on enhanced analysis of cost-drivers and cost-effectiveness is an opportunity for the PMPRB to provide more critical analysis of price, utilization, cost trends and other necessary analysis relevant to decision-makers through the NPDUIS. The challenge, however, will be to determine what additional analytical work in the area of pharmaceuticals through the NPDUIS could best contribute to the NPS.
To better align NPDUIS analysis with the needs of public policy decision-makers and address the challenges and opportunities that the NPS presents, the NPDUIS Steering Committee has planned to conduct a needs assessment of information and analysis needs with respect to pharmaceutical management and utilization. The purpose of the needs assessment is to determine what information stakeholders require to make informed decisions about strategic pharmaceutical management issues. The needs assessment will provide the basis for priority-setting for NPDUIS initiatives and will assist in determining whether further work is needed to refine or develop workable strategies in information-shy areas. Depending on the results, NPDUIS projects may be refined, or new ones added. NPDUIS projects are reported through the PMPRB Research Agenda, available on our Web site under Publications.
For current information on the involvement of the PMPRB and CIHI in NPDUIS, please visit their respective Web sites - at www.pmprb-cepmb.gc.ca and www.cihi.ca/drugs.
With the adoption of the 1987 amendments to the Patent Act (Act), Canada's Research Based Pharmaceutical Companies (Rx&D) made a public commitment that brand name manufacturers would increase their annual research-and-development (R&D) expenditure to 10% of sales revenue by 1996. [28]
Under the Act, the PMPRB monitors and reports on R&D spending, but has no regulatory authority over the amount or type of research spending by patentees. This chapter provides key statistics on the current state of pharmaceutical research investment in Canada.
Data Sources
The results presented here were derived from data patentees have submitted to the PMPRB. The Act requires each patentee to report its revenue from sales of drugs (including revenue from sales of non-patented drugs and from licensing agreements) and R&D expenditure in Canada related to medicines. Companies without sales of patented medicines need not report on R&D expenditure and, as new patents are granted and others expire, the set of companies required to file R&D data changes from year to year.
The Patented Medicines Regulations (Regulations) require that the R&D data submitted to the PMPRB be accompanied by a certificate stating that the submitted information is "true and correct". The Board does not audit submissions, but it does review submitted data for anomalies and inconsistencies, seeking corrections or clarifications from patentees where these are detected. To confirm that Board Staff has correctly interpreted submitted data, each patentee is given the opportunity to review and confirm the accuracy of its own R&D-to-sales ratio before publication of this report.
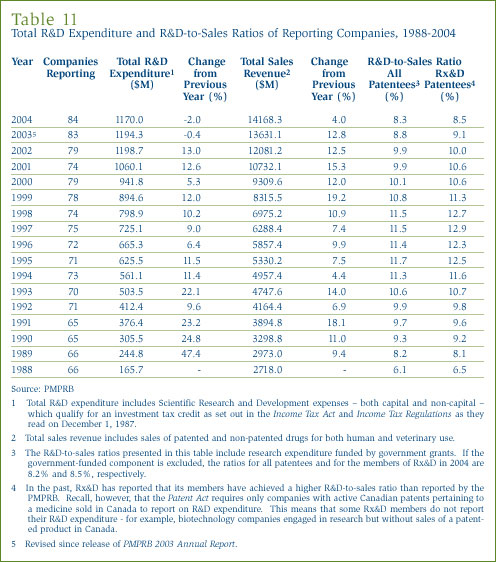
For 2004 a total of 84 companies selling human and veterinary drug products filed reports on their R&D expenditure. Of these, 37 were members of Rx&D.
Failure to File
In its 2003 Annual Report, the PMPRB reported that it was investigating the failure of Draxis Health Inc. to report information showing its expenditures on pharmaceutical R&D. This matter has now been concluded with the reporting of R&D expenditure information by Draxis Health Inc. for 2003.
Sales Revenue
For reporting purposes, sales revenue is defined to include all revenue from Canadian sales of medicines [29] and from licensing agreements.
As shown in Table 11, on page 32, patentees reported total sales revenue of $14.2 billion from Canadian sales of drugs in 2004, up 4% over 2003. Less than 1% of reported sales revenue was generated by licensing agreements. Sales revenue reported by Rx&D members totalled $11.8 billion, accounting for 83.1% of the total.
R&D Expenditure
Pursuant to Section 6 of the Patented Medicines Regulations, patentees are required to report R&D expenditure that would have been eligible for an Investment Tax Credit for scientific research and experimental development under the provisions of the Income Tax Act in effect on December 1, 1987. By this definition, R&D expenditure may include current expenditure, capital equipment costs and allowable depreciation expenses. Market research, sales promotions, quality control or routine testing of materials, devices or products and routine data collection are among the types of expenditure not eligible for an Investment Tax Credit, and are not to be included in patentees' filings.
As shown in Table 11, total 2004 R&D expenditure reported by patentees was $1,170.0 million, 2.0% less than in 2003. Rx&D members reported R&D expenditure of $1,008.3 million in 2004, accounting for 86.2% of all reported expenditure.
R&D-to-Sales Ratios
The ratio of R&D expenditure to sales revenue for the patented pharmaceutical industry was 8.3% in 2004. The ratio for members of Rx&D was 8.5%, down from 9.1% in the previous year.
Figure 18, on page 33, shows that R&D-to-sales ratios for all patentees and for Rx&D members have declined in recent years, after rising from 1988 to the mid-1990s. Whether taken over all patentees or restricted to Rx&D members, R&D-to-sales ratios in 2004 were the lowest seen since 1989.
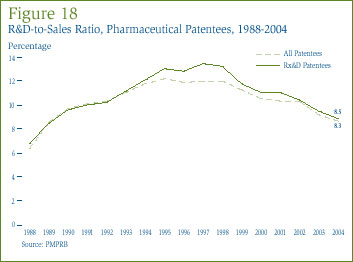
Table 13 in Annex 3, on page 49, provides details on the range of R&D-to-sales ratios. Of the 84 companies reporting in 2004, 66 had R&D-to-sales ratios of 10% or less in 2004. These companies accounted for 68.7% of total sales revenue.
Table 14 in Annex 3, starting on page 49, lists all reporting patentees and their R&D-to-sales ratios.
Current Expenditure by Type of Expenditure
Current R&D expenditure [30] was $1,124.2 million in 2004, accounting for 96.1% of total R&D expenditure. Capital equipment costs and allowable depreciation expenses made up 2.4% and 1.5% of total R&D expenditure, respectively.
Current Expenditure by Type of Research
Table 15 in Annex 3, on page 52, gives the allocation of 2004 current expenditure on basic research, applied and other qualifying R&D. Basic research is defined as work that advances scientific knowledge without a specific application in view. Patentees reported spending $221.7 million on basic research, representing 19.7% of current R&D expenditure. As shown in Figure 19, spending on basic research increased by 23% in 2004 relative to the previous year.
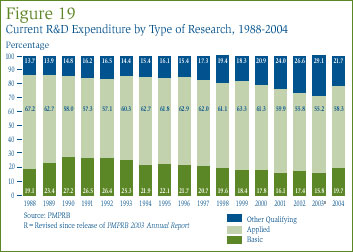
Applied research is directed toward some specific practical application, comprising research intended to improve in manufacturing processes, pre-clinical trials and clinical trials. Patentees reported spending $658.3 million on applied research, representing 58.3% of current R&D expenditure. Clinical trials accounted for 76.2% of applied research expenditure.
Other qualifying research (includes drug regulation submissions, bioavailability studies and Phase IV clinical trials) accounted for the remaining 21.7% of current expenditure in 2004.
Current Expenditure by R&D Performer and by Source of Funds
Patentees report expenditure on research they conduct themselves (intramural) and research performed by other establishments, such as universities, hospitals and other manufacturers (extramural). Table 16 in Annex 3, on page 53, shows that slightly more than one-half (55%) of expenditure was intramural. Research performed by other companies on behalf of patentees rose to 20.9% of current R&D expenditure in 2004, while the combined share of universities and hospitals was 13.2%.
In 2004, as in previous years, patentees funded almost all R&D expenditure with internal company funds. (Refer to Table 17 in Annex 3, on page 53, for further details.)
Current R&D Expenditure by Location
Tables 18 and 19 in Annex 3, on pages 53 and 54, show the current R&D expenditure by province. R&D expenditure increased in all parts of Canada except Ontario in 2004. As in previous years, expenditure was heavily concentrated in Ontario and Québec, these provinces accounting for 88.5% of total expenditure.
Research Agenda
The PMPRB's Research Agenda is developed each year as part of our annual planning process. It outlines current or upcoming projects which we are working on or will be undertaken in the near future. Initiatives that are currently, or may become, subject to public consultation are also indicated in the Research Agenda.
The National Prescription Drug Utilization Information System (NPDUIS), a partnership between the Canadian Institute for Health Information (CIHI) and the PMPRB, was launched in 2002. Under this initiative, the PMPRB has undertaken a number of research studies related to the utilization and management of pharmaceutical products. The NPDUIS research projects are reflected in the Research Agenda.
In addition, recent consultations on proposed amendments to the Patented Medicines Regulations and price increases for patented medicines are reflected in our Research Agenda.
Updates to the Research Agenda are published quarterly in the PMPRB's NEWSletter.
Timelines
In its second report to the Board in November 2000, the former Working Group on Price Review Issues [31] recommended the establishment of milestones and time frames for the price review process. Building on this recommendation, on the complex questions raised by new drugs that require expert review, on the novel submissions of patentees and on their requests for additional opportunities to provide more evidence and make further submissions, the PMPRB initiated a Timelines Review project.
In our 2003 Annual Report, we reported on the internal initiatives undertaken in preparation for further changes and future public consultation on the Timelines Review project. In addition to continuing with the implementation of these initiatives in 2004, we made further enhancements. Among others, we have published the schedule of meetings of the Human Drug Advisory Panel (HDAP) for 2005 and established deadlines for receipt of patentees' submissions for the HDAP's consideration. These steps were introduced to make the scientific review process more transparent and to increase its efficiency.
One area that was identified as having an impact on the timeliness of the price review process is the information that is filed by a patentee; in particular, the information required to begin the scientific review process. As part of the January 2005 NEWSletter, the PMPRB published a Notice and Comment on proposed amendments to the Patented Medicines Regulations. One of these amendments would require a patentee to file a product monograph. Comments on this and on other proposed amendments were to be forwarded to the Secretary of the Board no later than April 15, 2005. After having considered the submissions received, the Board will report next steps in this initiative and publish stakeholders' submissions, through its Research Agenda.
The next steps in the Timelines Review project involve developing timeframes for the stages of the review process and consulting with industry and other stakeholders.
We will report further to our stakeholders on this matter as the project evolves through articles in the NEWSletter and on our Web site.
Regulatory Amendments - Public Consultation
In its January 2005 NEWSletter, the Board called on stakeholders to participate in its consultation on proposed amendments to the Patented Medicines Regulations, 1994 (Regulations). Patentees' filing requirements with respect to the PMPRB are set out in the Regulations. The Regulations specify the information that patentees must file to the PMPRB in accordance with their obligations under the Patent Act, and the timeframes for doing so.
Since they were initially promulgated in 1988, the most substantive amendments to the Regulations occurred in 1994. The primary reason for the 1994 amendments was to bring the Regulations up to date with the latest version of the Patent Act.
A decade later, the Regulations need to be modernized in some areas to better reflect the information needs of the PMPRB to carry out its responsibilities under the Patent Act. The primary reasons for proposing amendments to the Regulations at this time are to support and improve the timeliness of the PMPRB's price reviews and to differentiate between proposed new filing requirements of patented drugs for human use and patented drugs for veterinary use.
The deadline for stakeholders' submissions on the proposed amendments to the Regulations was April 15, 2005. The Board will report next steps in this initiative and publish stakeholders' submissions, through its Research Agenda.
Price Increases for Patented Medicines: Public Consultation
In 2004, the PMPRB received information such as trade notices and reports from stakeholders, indicating that manufacturers of a significant number of patented medicines had informed customers of proposed price increases. While price increases in line with changes in the Consumer Price Index (CPI) are permitted under the PMPRB Price Guidelines, the number of reports of price increases raise questions.
Over the past decade, Canada has been able to achieve a level of price stability for patented medicines due to government policies such as the regulation activities of the PMPRB and the cost-containment strategies of public drug plans. The recent reports of price increases raise the question of whether Canada is on the verge of experiencing a change in pricing of patented medicines, bringing the past decade of price stability to an end. And, at the same time as these proposed increases were slated to occur in Canada, other countries were placing further limits on pharmaceutical prices.
In response to the reports of price increases, the PMPRB announced in November 2004 that it would review its Guidelines for price increases of patented medicines. [32] As a first step, the PMPRB began a dialogue with stakeholders on this issue through the release of a discussion paper on March 8, 2005. It explores the evolution of the Guidelines for price increases of patented medicines, highlights historical price trends and asks a series of questions on the issue of price increases for stakeholders to comment on.
The Board is considering the submissions it received from stakeholders at the close of the Notice and Comment period on May 9, 2005. We will report on next steps and updates on this project in an upcoming issue of the NEWSletter, and through our Research Agenda.
Two-Price System Project
Following the Board's acceptance of a Voluntary Compliance Undertaking (VCU) regarding the patented medicine Fasturtec, the PMPRB undertook to conduct a policy review into the practice of manufacturers of patented drugs maintaining high list prices that are not reflective of the average selling price in Canada.
In June 2004, the PMPRB concluded proceedings in the matter of Fasturtec by accepting a VCU from Sanofi-Synthelabo Canada Inc. (Sanofi). The VCU called for a significant decrease in the price of Fasturtec to $125 per vial, from $295. However, Sanofi indicated in its submission that it intended to maintain a high list price for Fasturtec, despite undertaking that no customer in Canada will pay more than the new reduced price. (The VCU and communiqué are available on our Web site under Publications; Voluntary Compliance Undertakings.)
Since this is a new issue for the PMPRB, Board Staff recommended to the Board that it initiate a review into the issue of publication of a high list price that is not reflective of the average selling price in Canada.
Related to this matter, questions about maintaining a two-price system have also been brought to the attention of the PMPRB in recent months through a few informal inquiries. No formal proposals have been made.
At a preliminary level, there may be some concerns with respect to consumer protection. The Board remains concerned that a two-price system may reduce transparency in patented drug prices in Canada.
We will be reporting as needed on this project through the PMPRB's Research Agenda. The Research Agenda is available on the PMPRB Web site under Publications.
Communications
The PMPRB Communications Program provides a management framework on all important aspects of the PMPRB's strategy and practices. It is an essential managerial and information service used in the decision-making and reporting processes.
The Communications Program includes the development and maintenance of our communications policies, plans and activities. The Secretariat manages the Communications Program, is responsible for responding to public enquiries and is accountable for the management, direction, development and dissemination of all communications activities, including media relations. It also participates in the collective management of the PMPRB activities.
We strive to foster a strong Communications plan. We continually cultivate an educational component to raise awareness and understanding of the mandate, role and jurisdiction of the Board.
In 2004, we remained committed to offering effective communications tools by promoting partnership for action between stakeholders and consumers through innovative modes of communication. We launched an enhanced Web site that is user-friendly and interactive. Among the site's new features is the opportunity to subscribe to our mailing list to receive paper or electronic copies of our publications. We also improved the site with a Frequently Asked Questions function that became quite popular with inquisitive visitors. The site's layout and presentation have been re-designed in order to ensure easier navigation to better meet the needs of Internet surfers.
Transparency, integrity and accessibility continue to be the central elements of our Communications Program.
The Board consists of not more than five members who serve on a part-time basis, appointed by the Governor-in-Council, including a Chairperson and Vice-Chairperson. The Chairperson is designated under the Patent Act as the Chief Executive Officer of the PMPRB with the authority and responsibility to supervise and direct its work. The Executive Director manages the work of the Staff. Senior Staff consists of the Executive Director, the Director of Compliance and Enforcement, the Director of Policy and Economic Analysis, the Director of Corporate Services, the Secretary of the Board and Senior Counsel.
Dr. Robert G. Elgie, Chairperson of the PMPRB since 1995, completed a ten-year mandate on March 7, 2005. Réal Sureau, Vice-Chairperson, has been assuming the functions of Chairperson of the PMPRB since March 8, 2005 and will ensure continuity until a permanent Chair is appointed. Mr. Sureau's ten-year mandate at the PMPRB ends in October 2005.
Dr. Brien Benoit was appointed to the Board on May 19, 2005 for a five-year term. Anthony Boardman completed a five-year mandate as Board Member in January 2004. He was reappointed for another five-year term on March 11, 2005. Ingrid Sketris completed her mandate as Board Member in May 2004.
Wayne Critchley, who has served the PMPRB over the last 15 years as Executive Director has retired and left the task in the capable hands of Barbara Ouellet, formerly Director of the Quality Care, Technology and Pharmaceuticals Division in the Health Policy Branch at Health Canada.
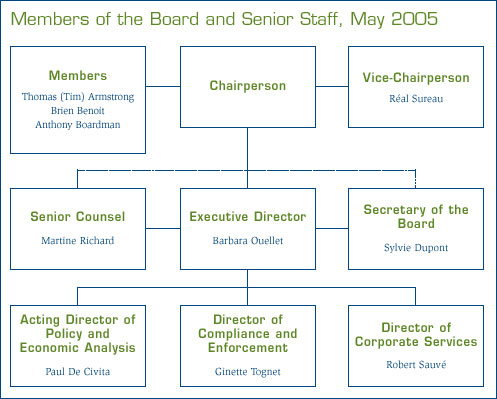
Chairperson: Robert G. Elgie
C.M., LL.B., M.D., F.R.C.S. (C), LL.D. (hon.)
Dr. Elgie was appointed Member and Chairperson of the Board in March 1995 and re-appointed in March 2000 to March 2005. Dr. Elgie completed his mandate with the PMPRB on March 7, 2005.
Dr. Elgie, a lawyer and neurosurgeon, Fellow of the Royal College of Surgeons (Neurosurgery), was the founder and first Director of Dalhousie University's Health Law Institute from 1991 to 1996. He was also the part-time Chair of the Workers' Compensation Board of Nova Scotia from 1992 to 1996. Dr. Elgie has taught at the Medical Schools of Queen's University and the University of Toronto, and has held several positions with the Scarborough General Hospital, including Chief of Medical Staff. In 1977, he was elected to the Ontario Legislative Assembly and subsequently served in several Cabinet positions. He resigned from the Ontario Legislature in September 1985 to become Chair of the Workers' Compensation Board of Ontario where he served until 1991. In October 2000, Dr. Elgie was appointed to the Ontario Press Council.
In May 2001, Dr. Elgie was awarded an honorary degree by Dalhousie University: Doctor of Laws, honoris causa, in recognition of his outstanding personal achievements. In January 2003, Dr. Elgie was appointed Member of the Order of Canada.
Vice Chairperson : Réal Sureau, F.C.A.
Mr. Sureau was appointed Member and Vice-Chairperson of the Board in October 1995 and re-appointed in October 2000 to October 2005.
Mr. Sureau assumed the functions of Chairperson of the PMPRB on March 8, 2005. He is a graduate of accountancy courses at Queen's University and McGill University and became a chartered accountant in 1963.
From 1957 until 1973 Mr. Sureau practiced public accounting and auditing in a regional firm. He then became Vice-President, Finance of Forex Inc. engaged in sawmill activities from 1973 to 1982. He subsequently moved as chief financial officer of the Canam Manac Group Inc., a North American leader in the manufacturing of steel trusses and semi-trailers until 1992. He then pursued his career as a business consultant and a corporate director.
Mr. Sureau was a director and member of several committees of the Québec Order of Chartered Accountants for which he served as Chairperson in 1995-1996. He was granted an honorary Fellowship in 1986.
Mr. Sureau is President of Sureau Management Limited. Since 1982, he has served on several boards of directors of reporting issuers and other private organizations, including Gaz Métro Inc. where he sits as a director, member of the committee on pension funds, and chairs their audit committee since 1995.
Members:
Thomas (Tim) Armstrong
Q.C., O. Ont.
Mr. Armstrong was appointed Member of the Board on October 3, 2002 to October 2007.
A lawyer, Mr. Armstrong has had a long career as a provincial public servant. He served as Chair of the Ontario Labour Relations Board (1974-1976), Deputy Minister of Labour (1976-1986), Agent General for Ontario in Tokyo (1986-1990), and Deputy Minister of Industry, Trade and Technology (1991-1992). He was advisor to the Premier of Ontario on Economic Development from 1992 to 1995. He has been Chief Representative for Canada to the Japan Bank for International Cooperation since 1996 and also serves as arbitrator and mediator in alternative dispute resolutions (ADR), specializing in labour relations.
Mr. Armstrong was awarded the Order of Ontario in 1995 in recognition of his contribution to public service in Ontario.
Brien G. Benoit
M.D., F.R.C.S. (C), F.A.C.S.
Dr. Benoit was appointed Member of the PMPRB in May 2005 to May 2010.
A Neurosurgeon, Dr. Benoit is on the Active Attending Staff of the Ottawa Hospital. Dr. Benoit is also Professor of Neurosurgery at the University of Ottawa. Throughout his career Dr. Benoit has held several administrative positions among which Chief of Staff of the Ottawa Civic Hospital, from 1996 to 1998; Program Director, Neurosurgery at the University of Ottawa, from 1995 to 2003; Chair of Neurosurgery at the University of Ottawa, from 1997 to 2003; and Deputy Surgeon-in-Chief at the Ottawa Hospital (Civic Campus), from 2002 to 2004. Dr. Benoit was also Chair of the Operating Room Committee at the Ottawa Hospital (Civic Campus), from 1993 to 2004.
Dr. Benoit has published extensively in leading academic journals. He has received several awards, including Best Surgical Teacher from the Department of Surgery of the University of Ottawa in 1991 and 2000.
In addition to being Fellow of the Royal College of Surgeons (Neurosurgery), Dr. Benoit is member of several professional associations including the Canadian Medical Association, the Ontario Medical Association, the Royal College of Physicians and Surgeons of Canada, and the American College of Surgeons, to name a few.
Anthony Boardman
B.A., Ph.D.
Dr. Boardman was appointed Member of the Board in January 1999 to January 2004. He was appointed for a second term in March 2005 to March 2010.
Dr. Boardman is the Van Dusen Professor of Business Administration in the Strategy and Business Economics Division of the Sauder School of Business at the University of British Columbia (UBC). He graduated from the University of Kent at Canterbury, England, (B.A., 1970) and Carnegie-Mellon University (Ph.D., 1975). Prior to taking up his position at UBC he was a professor at the Wharton School, University of Pennsylvania.
Dr. Boardman's current research interests include public-private partnerships, cost-benefit analysis and strategic management. Dr. Boardman has been a consultant to many private and public organizations including Vodafone, Stora Enzo, PricewaterhouseCoopers, the Treasury of New Zealand and all levels of government in Canada. He has taught executive programs throughout the world, and has won a number of teaching awards, including the Alan Blizzard award.
During his career, Dr. Boardman has published many articles in leading academic journals. Currently, he is working on the third edition of Cost-Benefit Analysis: Concepts and Practice, which is published by Prentice Hall.
Ingrid S. Sketris
BSc(Phm), Pharm.D., MPA(HSA)
Dr. Sketris was appointed Member of the Board in May 1999 to May 2004.
Dr. Sketris is a Professor at the College of Pharmacy and School of Health Services Administration and a Professor of the Department of Community Health and Epidemiology, Dalhousie University. She is a consultant to the pharmacy department of the Queen Elizabeth II Health Sciences Centre, Halifax. Since 2000, Dr. Sketris holds a Chair in health services and nursing from the Canadian Health Services Research Foundation/Canadian Institutes of Health Research (cosponsored by the Nova Scotia Health Research Foundation).
She is a graduate of the University of Toronto (BSc(Phm), 1977), University of Minnesota (Pharm.D,1979), University of Tennessee Center for the Health Sciences (Residency in Clinical Toxicology/Pharmacy Practice, 1980) and Dalhousie University (MPA(HSA) 1989).
Dr. Sketris is a fellow of the Canadian Society of Hospital Pharmacists and the American College of Clinical Pharmacy. She was a member of the scientific advisory panel of the Canadian Coordinating Office for Health Technology Assessment (CCOHTA) from 1996-1998. Dr. Sketris' research interests include examining the impact of changes in Pharmacare policy and the use of drugs and health services particularly related to the population of Nova Scotia.
Dr. Sketris currently sits as the PMPRB representative on a committee of the Canadian Institute for Health Information (CIHI) examining the use of the Anatomical Therapeutic Chemical (ATC) Classification System and Defined Daily Dose (DDD) for analytical purposes.
Dr. Sketris has numerous publications in the area of transplantation therapeutics and pharmacoepidemiology.
The PMPRB operated with a budget of $5,417,000 in 2004-2005 and an approved staff level of 44 employees. The budget included $424,000 for the Therapeutic Access Strategy [33] (TAS) initiative and $832,000 for the National Prescription Drug Utilization Information System (NPDUIS) [34] project.

Additional information on the PMPRB budget is available on our Web site under Reports to Parliament.
We inform our stakeholders regularly through our publications. Our Annual Report and the NEWSletter are published at regular intervals throughout the year while other publications are released in response to program and corporate requirements.
Publications / Release Date
January 2004 - March 2005
- Advance Ruling Certificate
- Annual Report / June
- CPI-Adjustment Factors / April
- Hearings
- Nicoderm - Hoechst Marion Roussel Canada Inc. / April 1999 (ongoing)
- Fasturtec, Sanofi-Synthelabo Canada Inc. / May (complete)
- Dovobet, LEO Pharma Inc. / November (ongoing)
- Evra, Janssen-Ortho Inc. / December (complete)
- NEWSletter / Quarterly
- Notice and Comment
- Schedule 7 of the Compendium of Guidelines, Policies and Procedures - Comparable Dosage Forms, NEWSletter / January
- Proposed Amendments to the Patented Medicines Regulations - NEWSletter / January 2005
- Price Increases for Patented Medicines - NEWSletter (article) / March 2005
- Patented Medicines
- Reported to the PMPRB in 2004 (including the review status for each drug) / Monthly
- Reports on New Patented Drugs:
- Aerius / June
- Alphagan / September
- Avodart / October
- Bextra / March
- BLES / June
- Bondronat / April 2005
- Cetrotide / January 2005
- Crestor / January
- Ebixa / January 2005
- Ezetrol / September
- Fasturtec / August
- Gadovist / January 2005
- Gynazole / January 2005
- Hectorol / September
- Infergen / September
- Iressa / October
- Kineret / September
- Lantus / February 2005
- Pegasys / June
- Valcyte / July
- Viread / January 2005
- Xatral / May
- Xigris / January
- Zavesca / January 2005
- Research Agenda [35] / January
- Speech Series
- Patented Medicines and Pricing Issues: Latest Trends and Developments / March
- Pharmaceutical Price Controls in Canada / May
- The Future of Price Controls - Maintaining the Balance / November
- Drug Prices in Canada and the U.S.: More Than Meets the Eye? / January 2005
- Introductory Remarks to the Standing Committee on Health - on Main Estimates / April 2005
- Voluntary Compliance Undertakings
- One-Alpha, LEO Pharma Inc. / May
- Fasturtec, Sanofi-Synthelabo Canada Inc. / June
- Prolastin, Bayer Inc. / July
- Starnoc, Servier Canada / July
- Busulfex, EPS Pharma Inc./ November
- Evra, Janssen-Ortho Inc. / February 2005
- Paxil CR, GlaxoSmithKline Inc. / March 2005
- Tamiflu, Hoffmann-La Roche Limited / March 2005
This glossary is included for the convenience of the reader. For more detailed information and definitions please refer to the Patent Act, the Patented Medicines Regulations, the PMPRB Compendium of Guidelines, Policies and Procedures and the Food and Drugs Regulations, or contact the PMPRB.
Active Ingredient:
Chemical or biological substance responsible for the claimed pharmacologic effect of a drug product. (Ingrédient actif)
Advance Ruling Certificate (ARC):
A non-binding certificate may be issued pursuant to subsection 98(4) of the Patent Act at the request of a patentee when the Board is satisfied that the price or proposed price of the medicine would not exceed the maximum non-excessive price under the Board's Guidelines. (Certificat de décision préalable)
ATC:
Anatomical Therapeutic Chemical [ATC] classification system, developed and maintained by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology, divides drugs into different groups according to their site of action and therapeutic and chemical characteristics. This system is used by the PMPRB as a guide for selecting comparable medicines for purposes of price review. (ATC)
Dedication of Patent:
A practice whereby a patentee notifies the Commissioner of Patents that it has surrendered its rights and entitlements flowing from the patent for the benefit of the public to use and enjoy. (Cession d'un brevet)
NB: As of January 30, 1995, the Board does not recognize dedication of patent as a means to remove the medicine from its jurisdiction. (See PMPRB Bulletin 17, page 3.)
Drug Identification Number (DIN):
A registration number that the Health Protection Branch of Health Canada assigns to each prescription and non-prescription drug product marketed under the Food and Drugs Regulations. The DIN is assigned using information in the following areas: manufacturer of the product; active ingredient(s); strength of active ingredient(s); pharmaceutical dosage form; brand/trade name; and route of administration. (Numéro d'identification de drogue)
Drug Product:
A particular presentation of a medicine characterized by its pharmaceutical dosage form and the strength of the active ingredient(s). (Produit médicamenteux)
Drug Product, Existing:
An existing drug product is a DIN for which a benchmark price has been established in accordance with the Board's Guidelines. (See Chapter 1, subsection 3.3 of the Compendium of Guidelines, Policies and Procedures.) (Produit médicamenteux existant)
Drug Product, New:
A new drug product is one for which the introductory price is under review. Patented drug products are considered new in the year during which they are first introduced on the market in Canada or the year they receive their first patent(s) if previously marketed. For price review purposes, new drug products for a given year are those introduced between December 1, of the previous year and November 30, of the reporting year. Because of the filing requirements under the Patented Medicines Regulations and the manner of calculating benchmark prices, drug products introduced in December are considered to have been introduced in the following year. (See Chapter 1, subsection 3.2 of the Compendium of Guidelines, Policies and Procedures.) (Produit médicamenteux nouveau)
Emergency Drug Release (EDR) Program:
See Special Access Program.
Generic Product:
A drug product with the same active ingredient, strength and dosage form of a brand name drug product. (Produit générique)
Investigational New Drug (IND):
A drug that has been authorized for clinical evaluation (i.e. testing on humans) by Health Canada but that is not yet approved for sale for the indication under study. (Drogue de recherche)
Licence, Compulsory:
Referred to in subsection 79(1) of the Patent Act, means a licence granted by the Commissioner of Patents, before December 20, 1991, in accordance with subsection 39(4) of the Patent Act, R.S., 1985, c. P-4 that has been continued pursuant to subsection 11(1) of the Patent Act Amendment Act, 1992 which permits the licencee to import, make, use or sell a patented invention pertaining to a medicine. Royalties payable are determined by the Commissioner of Patents who sets the terms of licences pursuant to subsection 39(5) of the Patent Act. (Licence obligatoire)
Licence, Voluntary:
A contractual agreement between a patent holder and a licensee under which the licensee is entitled to enjoy the benefit of the patent or to exercise any rights in relation to the patent for some consideration (i.e., royalties in the form of a share of the licensee's sales.) (Licence volontaire)
Medicine:
Any substance or mixture of substances made by any means, whether produced biologically, chemically, or otherwise, that is applied or administered in vivo in humans or in animals to aid in the diagnosis, treatment, mitigation or prevention of disease, symptoms, disorders, abnormal physical states, or modifying organic functions in humans and or animals, however administered. For greater certainty, this definition includes vaccines, topical preparations, anaesthetics and diagnostic products used in vivo, regardless of delivery mechanism (e.g. transdermal, capsule form, injectable, inhaler, etc.). This definition excludes medical devices, in vitro diagnostic products and disinfectants that are not used in vivo. (See Compendium of Guidelines, Policies and Procedures, Introduction, subsection 1.5.) (Médicament)
Notice of Compliance (NOC):
A notice in respect of a medicine issued by the Health Products and Food Branch of Health Canada under section C.08.004 of the Food and Drugs Regulations. The issuance of an NOC indicates that a drug product meets the required Health Canada standards for use in humans or animals and that the product is approved for sale in Canada. (Avis de conformité)
Patent:
An instrument issued by the Commissioner of Patents in the form of letters patent for an invention that provides its holder with a monopoly limited in time, for the claims made within the patent. A patent gives its holder and its legal representatives, the exclusive right of making, constructing and using the invention and selling it to others to be used. (Brevet)
Patented Medicine Price Index (PMPI):
The PMPI has been developed by the PMPRB as a measure of average year-over-year change in the transaction prices of patented drug products sold in Canada, based on the price and sales information reported by patentees. (Indice des prix des médicaments brevetés)
Patentee:
As defined by subsection 79(1) of the Patent Act, "the person for the time being entitled to the benefit of the patent for that invention (pertaining to a medicine) and includes, where any other person is entitled to exercise any rights in relation to that patent other than under a licence continued by subsection 11(1) of the Patent Act Amendment Act, 1992, that other person in respect of those rights;" (Breveté)
Pending Patent:
An application for a patent that has not yet been issued. (Brevet en instance)
NB: In cases where a medicine is sold before a patent is issued, it is the Board's policy once the patent is issued, to review the price of the medicine as of the date on which the patent application was laid open for public inspection. (See PMPRB Bulletin 15, page 7.)
Research and Development (R&D):
Basic or applied research for the purpose of creating new, or improving existing, materials, devices, products or processes (e.g. manufacturing processes). (Recherche et développement)
Research and Development-Applied Research:
Work that advances scientific knowledge with a specific practical application in view such as creating new or improved products or processes through manufacturing processes or through preclinical or clinical studies. (Recherche et développement-recherche appliquée)
Research and Development-Basic Research:
Work that advances scientific knowledge without a specific application in view. (Recherche et développement-recherche fondamentale)
Research and Development-Clinical Research:
The assessment of the effect of a new medicine on humans. It typically consists of three successive phases, beginning with limited testing for safety in healthy humans then proceeding to further safety and efficacy studies in patients suffering from the target disease. (Recherche et développement-recherche clinique)
Research and Development-Preclinical Research:
Tests on animals to evaluate the pharmacological and toxicological effects of medicines. (Recherche et développement-recherche pré-clinique)
Research and Development-Other Qualifying:
Includes eligible research and development expenditures that cannot be classified into any of the preceding categories of "type of research and development". It includes drug regulation submissions, bioavailability studies and Phase IV clinical trials. (Recherche et développement-Autres R-D admissibles)
Research and Development Expenditures:
For the purposes of the Patented Medicines Regulations, 1994, in particular sections 5 and 6, research and development includes activities for which expenditures would have qualified for the investment tax credit for scientific research and experimental development under the Income Tax Act as it read on December 1, 1987. (Dépenses en recherche et développement)
Current Research and Development Expenditures:
consist of the following non-capital expenses that are directly related to research work: (a) wages and salaries, (b) direct material, (c) contractors and sub-contractors, (d) other direct costs such as factory overhead, (e) payments to designated institutions, (f) payments to granting councils and (g) payments to other organizations. These elements are described in greater detail in the Patentees' Guide to Reporting - Form 3 available from the PMPRB website under the heading of "Legislation, Regulations and Guidelines." (Dépenses courantes de recherche et développement)
Special Access Program (SAP):
A program operated by Health Canada to give practitioners access to drugs that are not approved or otherwise available for sale in Canada. (Formerly the EDR Program.) (Programme d'accès spécial)
Voluntary Compliance Undertaking (VCU):
A written undertaking by a patentee to adjust its price to conform with the PMPRB's Excessive Price Guidelines (see Chapter 1 of the Compendium of Guidelines, Policies and Procedures). Pursuant to the Board's Compliance and Enforcement Policy (see Chapter 2, section 7) the Chairperson may approve a VCU in lieu of issuing a Notice of Hearing if it is consistent with the Patent Act and the policies of the Board and in the public interest. Under the Board's Compliance and Enforcement Policy, a VCU can also be submitted following the issuance of a Notice of Hearing. A VCU submitted at this point must be approved by the Board. The Board reports publicly on all VCUs approved by the Chairperson or the Board. (Engagement de conformité volontaire)
This section provides the list of acronyms used in the Annual Report and frequently referenced by the PMPRB and its stakeholders.
ARC: Advance Ruling Certificate
ATC: Anatomical Therapeutic Chemical classification system
ATP: Average Transaction Price
CAC: Consumers' Association of Canada
CCOHTA: Canadian Coordinating Office for Health Technology Assessment
CDR: Common Drug Review
CEDAC: Canadian Expert Drug Advisory Committee
CGPA: Canadian Generic Pharmaceutical Association
CIHI: Canadian Institute for Health Information
CPI: Consumer Price Index
DDD: Defined Daily Dose
DIN: Drug Identification Number
DPD: Drug Product Database (Health Canada)
DVA: Department of Veterans Affairs (U.S.)
FDA: Food and Drugs Act (Canada)
FPG: First Patent Granted
F/P/T: Federal/Provincial/Territorial
FSS: Federal Supply Schedule (U.S.)
GDP: Gross Domestic Product
HDAP: Human Drug Advisory Panel
IPC: International Price Comparison
IPPI: Industrial Product Price Index
MIP: Median International Price
MNE: Maximum Non-Excessive (price)
MOU: Memorandum of Understanding
NAS: New Active Substance
NDMAC: Nonprescription Drug Manufacturers Association of Canada
NOC: Notice of Compliance
NPDUIS: National Prescription Drug Utilization Information System
NPS: National Pharmaceutical Strategy
NPSS: Non-Patented Single Source (drugs)
ODB: Ontario Drug Benefit Plan
OECD: Organisation for Economic Cooperation and Development
OTC: Over-the-counter
PMPI: Patented Medicine Price Index
PMPRB: Patented Medicine Prices Review Board
PMQI: Patented Medicine Quantity Index
R&D: Research and Development
Rx&D: Canada's Research Based Pharmaceutical Companies
SAP: Special Access Program (Health Canada)
TCC: Therapeutic Class Comparison
TPD: Therapeutic Products Directorate (Health Canada)
VCU: Voluntary Compliance Undertaking
WHO: World Health Organization
Criteria for Commencing an Investigation
A price is considered to be within the Guidelines unless it meets the criteria for commencing an investigation. The criteria represent the standards the Board applies in order to allocate its resources to investigations as efficiently as possible. Their existence should not be construed as indicating that the Board accepts any deviation from the Guidelines. The Board is satisfied that its criteria assure all significant cases of pricing outside the Guidelines will be subject to investigation. In most instances where a price exceeds the maximum allowable price by an amount too small to trigger an investigation in one year, it is offset by a price below that which is permitted by the Guidelines the following year. The Board expects the prices of all patented medicines to be within the Guidelines and evidence of persistent pricing outside the Guidelines, even by a small amount, may be used as a criterion for commencing an investigation.
Criteria for Commencing an Investigation
Board Staff will commence an investigation into the price of a patented drug product when any of the following criteria are met:
New Drug Products
- The introductory price is 5% or more above the maximum non-excessive price;
- Excess revenues in the introductory period are $25,000 or more; or
- Complaints with significant evidence.
Existing Drug Products
- A price is 5% or more above the maximum non-excessive price and there are cumulative excess revenues of $25,000 or more over the life of the patent after January 1, 1992;
- Cumulative excess revenues are $50,000 or more over the life of the patent after January 1, 1992; or
- Complaints with significant evidence.
For more information on the Criteria for Commencing an Investigation, please consult Schedule 5 of the Compendium of Guidelines, Policies and Procedures available on our Web site under Legislation, Regulations, Guidelines.
Patented Drug Products Introduced in 2004
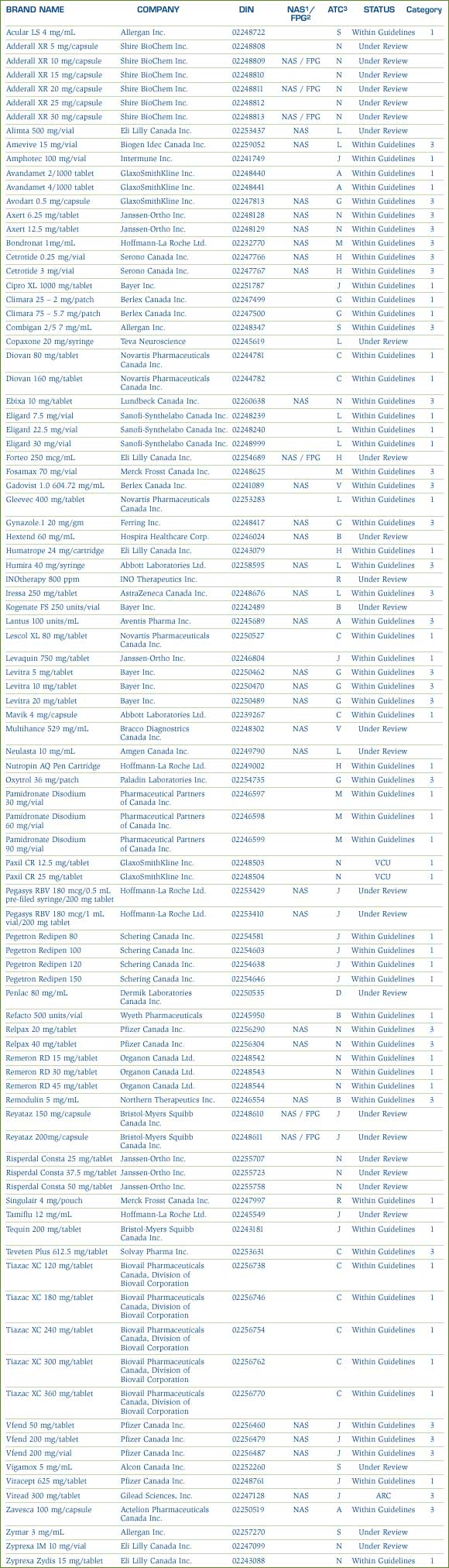
The Board's Guidelines establish three categories of new patented drug products for purposes of conducting introductory price reviews.
- Category 1 - a new DIN of an existing or comparable dosage form of an existing medicine, usually a new strength of an existing drug (line extension)
- Category 2 - the first drug to treat effectively a particular illness or which provides a substantial improvement over existing drug products, often referred to as "breakthrough" or "substantial improvement".
- Category 3 - a new drug or new dosage form of an existing medicine that provides moderate, little or no improvement over existing medicines.
For complete definitions of the categories, refer to the Compendium of Guidelines, Policies and Procedures, Chapter 3, section 3.
- NAS: New Active Substance
- FPG: First Patent Grant
- ATC: Anatomical Therapeutic Chemical Classification System
Research and Development
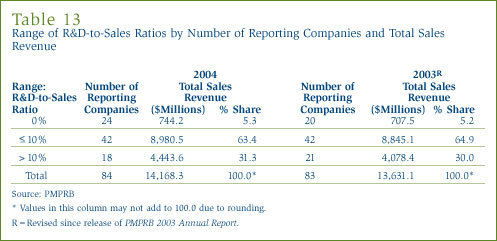
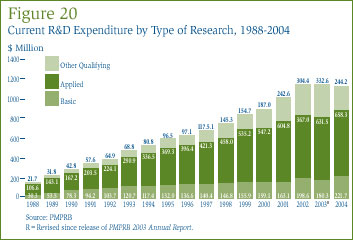
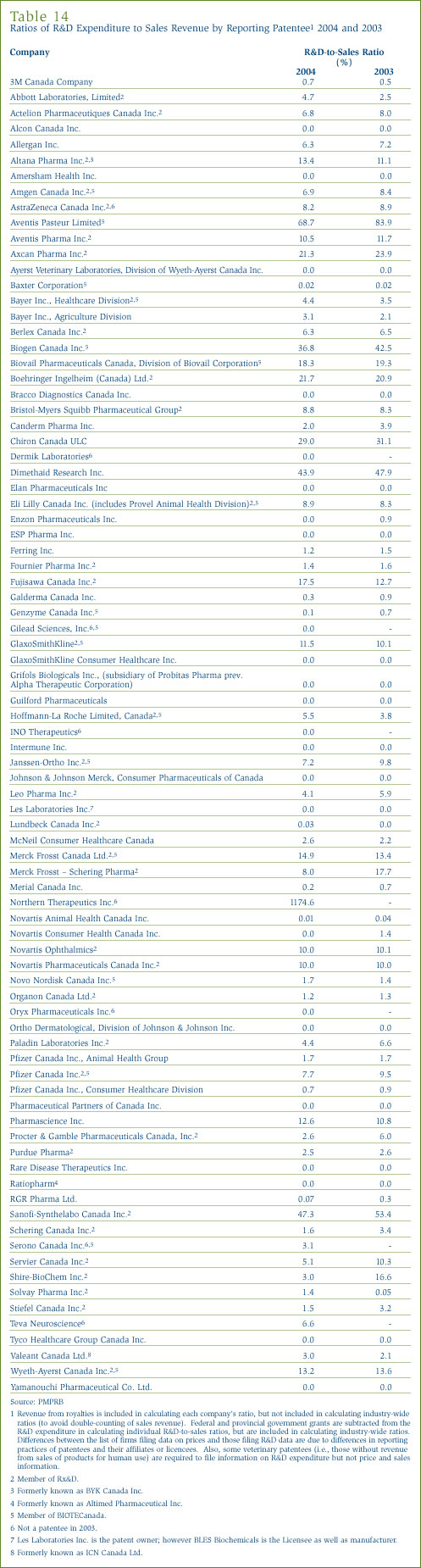
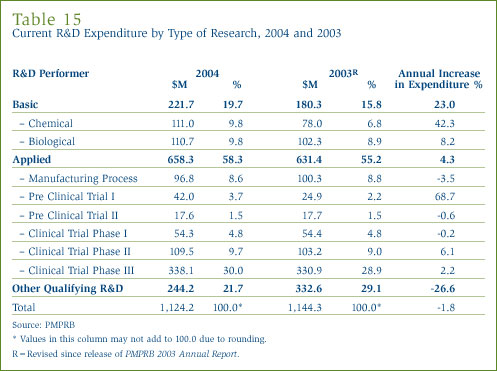
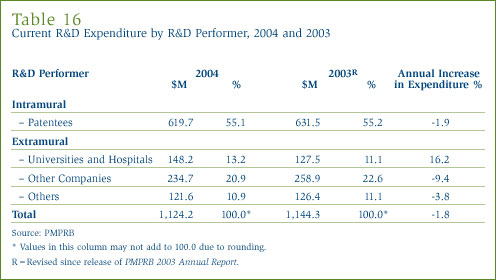
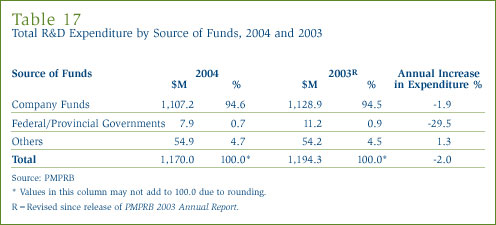
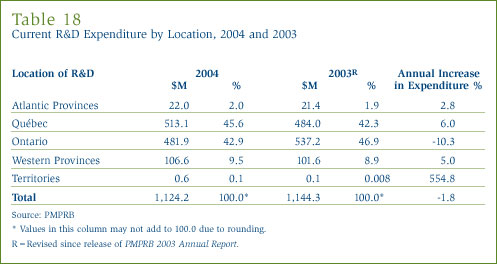
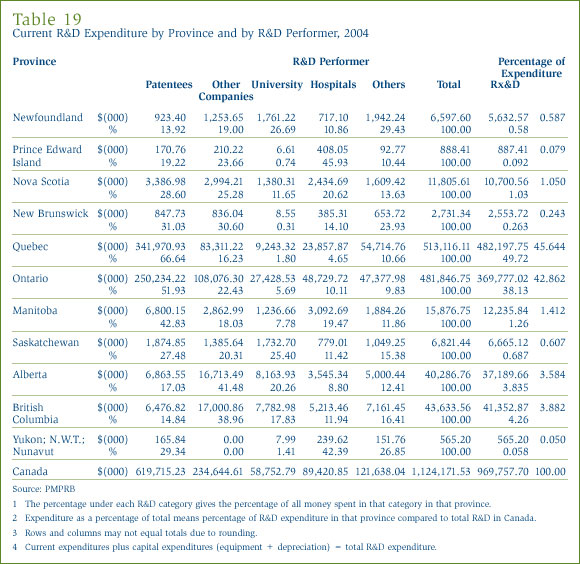
Download the Patented Drug Products for Human Use and Canadian Patentees - January 1, 2004 - December 31, 2004 in PDF format...
[1] The Health Portfolio contributes to specific dimensions of improving the health of Canadians. It comprises Health Canada and three agencies, the Canadian Institutes of Health Research, the Hazardous Materials Information Review Commission and the Patented Medicine Prices Review Board.
[2] Annual Drug Submission Performance Report, January-December 2004, Therapeutic Products Directorate, Health Canada.
[3] This drug, which was on the market before 2004, came under the PMPRB's jurisdiction in 2004 with the issuance of a patent.
[4] For purposes of conducting introductory price reviews, the PMPRB categorizes new drug products as follows:
- Category 1 - a new DIN of an existing or comparable dosage form of an existing medicine, usually a new strength of an existing drug (line extension).
- Category 2 - the first drug to treat effectively a particular illness or which provides a substantial improvement over existing drug products, often referred to as "breakthrough" or "substantial improvement".
- Category 3 - a new drug or new dosage form of an existing medicine that provides moderate, little or no improvement over existing medicines.
For complete definitions of the categories, refer to the Compendium of Guidelines, Policies and Procedures, Chapter 3, section 3.
[5] Because of the timing of the filing requirements under the Patented Medicines Regulations and the manner of calculating benchmark prices, drug products introduced or patented in December are considered to be new patented products in the following year.
[6] Beginning with the year 1999, the calculation of manufacturers' sales of all drugs and patented drugs includes the sales of drug products for human use only.
[7] Previous Annual Reports have produced statistical evidence of little change in patented drug prices while sales growth among patented drugs was 15% or more.
[8] Studies conducted by the PMPRB of public pharmaceutical insurance plans indicate that increased utilization of existing and new drugs accounts for most of the recent growth in expenditures. PMPRB, Provincial Drug Plan Overview Report: Pharmaceutical Trends, 1995/96 -1999/00, September 2001.
[9] IMS Health, Canadian Hospital and Pharmacy Audit.
[10] It should be noted that shares of sales by ATC group for all drugs in Canada may differ from shares obtained for patented drugs.
[11] These groups have been combined for reasons of confidentiality.
[12] See the PMPRB's A description of the Laspeyres methodology used to construct the Patented Medicine Price Index (PMPI), March 1997, revised June 2000, for a detailed explanation of the PMPI. The PMPI measures the overall change in the prices of existing patented drug products, and is constructed by taking a sales-weighted average of rates of price change at the level of individual products. It is not designed to measure the effects of changes in the quantities of drugs consumed or substitution among drugs (for example, the use of newer drugs in place of older, possibly less costly drugs) on sales. As of the 1999 Annual Report, the PMPI encompasses prices of patented drugs for human use only.
[13] 1992 is the only year in which the PMPI rose at a faster rate than the CPI. To facilitate and encourage compliance by patentees, the PMPRB's CPI-adjusted methodology uses the forecast rate of CPI inflation published by the Department of Finance. The forecast CPI inflation rate for 1992 had been 3.2%, but the actual rate was 1.5%. For a full explanation of the CPI-adjusted methodology, please refer to Schedule 4 of the PMPRB's Compendium of Guidelines, Policies and Procedures.
[14] Statistics Canada, CANSIM, Series V735319. For 2004 as a whole, consumers paid an average of 1.9% more than they did in 2003 for the goods and services included in the CPI basket. This was a smaller increase than the 2.8% annual average rise measured in 2003. According to Statistics Canada the main contributors to this slowdown were automotive vehicle insurance premiums, natural gas, cigarettes, computer equipment and supplies and purchase and leasing of automotive vehicles.
[15] These groups have been combined for reasons of confidentiality.
[16] The methodology used by the PMPRB in conducting foreign price comparisons can be found in the Compendium of Guidelines, Policies and Procedures and in two papers published in 2002 entitled Foreign Price Trends for Patented Medicines and Verification of Foreign Patented Drug Prices.
[17] The PMPRB performs all currency conversions for a given period using a simple average of spot exchange rates recorded in the preceding 36 months. This approach has a smoothing effect, limiting the influence of transitory exchange rate adjustments on Canadian-to-foreign price comparisons. It also has the property of phasing-in the effects of long-term exchange rate movements. Because of this, a long-term appreciation or depreciation of the Canadian dollar may continue to produce adjustments in Canadian-to-foreign price ratios up to three years after the exchange rate shift has taken place.
[18] The kroner has a great deal of leverage in this regard, because Swedish prices often emerge as median international prices. The same is true of the euro, being the currency in which French, Italian and German prices are reported. Movements of the U.S. dollar, in contrast, have little impact on the average ratio, since U.S. prices tend to be at the upper end of the international price distribution and hence are seldom median international prices.
[19] The pharmaceutical industry in the U.S. has argued that the publicly available prices in that country do not reflect actual prices because of confidential discounts and rebates. Effective January 2000, and following public consultation, the PMPRB began including prices listed in the U.S. Federal Supply Schedule (FSS) in calculating the average U.S. price of patented drugs. The FSS prices are negotiated between manufacturers and the U.S. Department of Veterans' Affairs. They are typically less than other publicly available U.S. prices reported to the PMPRB by manufacturers.
[20] Like the PMPI, the PMQI is calculated using a chained Laspeyres index formula, with ratios of physical quantities in successive periods replacing the price ratios of the PMPI. Here again, the aggregate value of the index is obtained as a revenue-weighted average of ratios at the level of individual products. Since the PMQI covers only patented drugs it should not be taken to represent utilization trends in the overall pharmaceutical market.
[21] These groups have been combined for reasons of confidentiality.
[22] Considering the dominant role of imports as a source of drug supplies, as well as the role of exports and generics in Canadian drug manufacturing, the PMPRB will no longer use the IPPI (Pharma) as an indicator of the prices at which brand name drugs are sold to Canadians.
[23] Statistics Canada, CANSIM, Series V768221 and V768217
[24] Statistics Canada, CANSIM, Series V1576093. More exactly, the IPPI (Pharma) tracks the ex-factory prices of drugs produced in Canada. By design, the IPPI (Pharma) and the PMPI are very different indicators. Drugs produced for export are included in the calculation of the IPPI (Pharma), whereas drugs imported into Canada do not figure in its calculation. The PMPI is designed to measure trends in ex factory prices of patented drugs, most of which are manufactured abroad. The IPPI (Pharma), on the other hand, is arguably dominated by the ex-factory prices of Canadian-produced generic products.
[25] IMS Health's Retail Drug Monitor, December 2004 (www.imshealth.com). IMS Health's Retail Drug Monitor covers direct and indirect pharmacy (purchases that are direct from the manufacturing company, or indirect through a wholesaler) channel purchases from wholesalers and manufacturers in 13 key countries. Sales figures are at ex-manufacturer prices and include all prescription and certain over-the-counter data. Figures include sales from the hospital sector in Japan and mail order in the U.S. These 13 countries account for over two thirds of the world market. The 13 countries include Argentina, Australia, Brazil, Canada, France, Germany, Italy, Japan, Mexico, New Zealand, Spain, the U.K. and the U.S.
[26] This growth rate is not the same as PMPRB reported growth rate (Table 6, on page 19) because IMS Health's Retail Drug Monitor data covers only sales to pharmacies.
[27] The OECD defines "pharmaceutical expenditure" as "total expenditure on pharmaceutical and other medical non-durables." This comprises "medical preparations, branded and generic medicines, drugs, patent medicines, serums, and vaccines, vitamins and minerals and oral contraceptives." It also includes non-pharmaceutical items such as toothpaste and condoms. The statistics reported encompass expenditure by both private and public sectors. Pharmaceutical expenditure may or may not include the value of drugs dispensed in hospitals, depending on the country. In reporting results for Canada, the OECD uses estimates of the Canadian Institute for Health Information (CIHI) for pharmaceutical expenditure. CIHI's estimates do not include the value of drugs dispensed by hospitals.
[28] As published in the Regulatory Impact Assessment Statement (RIAS) of the Patented Medicines Regulations, 1988, published in the Canada Gazette Part II, Vol. 122, No. 20 - SOR/DORS/88-474.
[29] Sales of drugs for both human and veterinary use are included for the purpose of this section of the report.
[30] Current R&D expenditure consists of non-capital expenses directly related to research, including (a) wages and salaries, (b) direct material, (c) contractors and sub-contractors, (d) other direct costs such as factory overhead, (e) payments to designated institutions, (f) payments to granting councils and (g) payments to other organizations. These elements are described in more detail in the Patentees Guide to Reporting - Form 3, available from the PMPRB Web site under the heading Legislation, Regulations and Guidelines.
[31] The Working Group on Price Review Issues was established in 1998 following the PMPRB's release of its Road Map for the Next Decade. Composed of 12 members representing the PMPRB's stakeholder groups, the Working Group's mandate was to review, analyze and report on three issues: the use of the U.S. Department of Veterans Affairs formulary prices in the international price comparison; the price review process for new patented drug products; and category 3 drug prices. The Working Group completed its work in October 2002. The Working Group's reports and other relevant materials are available on the PMPRB Web site under Working Group on Price Review Issues.
[32] See The Future of Price Controls - Maintaining the Balance, by Dr. Robert G. Elgie, PMPRB Chairperson, in a speech to PHARMAC 2004, available on the PMPRB Web site under Publications; Speeches; Speech Series 2004.
[33] The Therapeutic Access Strategy is a Health Canada initiative aimed at helping Canadians maintain and improve their health by ensuring that human drugs and other therapeutic products are safe, of high quality, therapeutically effective, appropriately used and accessible in a timely and cost-effective fashion. The PMPRB received funding through the TAS for its timelines initiative. For more information on this initiative, see page 34.
[34] For more information on the National Prescription Drug Utilization Information System (NPDUIS), see page 30.
[35] The Research Agenda is published in the NEWSletter and posted on our Web site under Publications; Research Agenda.